| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Dipeptidyl peptidase 8 |
|---|
| Ligand | BDBM50214710 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_447608 (CHEMBL895495) |
|---|
| IC50 | 5800±n/a nM |
|---|
| Citation |  Koo, KD; Kim, MJ; Kim, S; Kim, KH; Hong, SY; Hur, GC; Yim, HJ; Kim, GT; Han, HO; Kwon, OH; Kwon, TS; Koh, JS; Lee, CS Synthesis, SAR, and X-ray structure of novel potent DPPIV inhibitors: oxadiazolyl ketones. Bioorg Med Chem Lett17:4167-72 (2007) [PubMed] Article Koo, KD; Kim, MJ; Kim, S; Kim, KH; Hong, SY; Hur, GC; Yim, HJ; Kim, GT; Han, HO; Kwon, OH; Kwon, TS; Koh, JS; Lee, CS Synthesis, SAR, and X-ray structure of novel potent DPPIV inhibitors: oxadiazolyl ketones. Bioorg Med Chem Lett17:4167-72 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Dipeptidyl peptidase 8 |
|---|
| Name: | Dipeptidyl peptidase 8 |
|---|
| Synonyms: | DPP8 | DPP8_HUMAN | DPRP-1 | DPRP1 | Dipeptidyl peptidase 8 (DPP-8) | Dipeptidyl peptidase 8 (DPP8) | Dipeptidyl peptidase 8/9 | Dipeptidyl peptidase IV-related protein 1 | Dipeptidyl peptidase VIII | Dipeptidyl peptidase VIII (DDP-VIII) | Prolyl dipeptidase DPP8 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 103342.62 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q6V1X1 |
|---|
| Residue: | 898 |
|---|
| Sequence: | MWKRSEQMKIKSGKCNMAAAMETEQLGVEIFETADCEENIESQDRPKLEPFYVERYSWSQ
LKKLLADTRKYHGYMMAKAPHDFMFVKRNDPDGPHSDRIYYLAMSGENRENTLFYSEIPK
TINRAAVLMLSWKPLLDLFQATLDYGMYSREEELLRERKRIGTVGIASYDYHQGSGTFLF
QAGSGIYHVKDGGPQGFTQQPLRPNLVETSCPNIRMDPKLCPADPDWIAFIHSNDIWISN
IVTREERRLTYVHNELANMEEDARSAGVATFVLQEEFDRYSGYWWCPKAETTPSGGKILR
ILYEENDESEVEIIHVTSPMLETRRADSFRYPKTGTANPKVTFKMSEIMIDAEGRIIDVI
DKELIQPFEILFEGVEYIARAGWTPEGKYAWSILLDRSQTRLQIVLISPELFIPVEDDVM
ERQRLIESVPDSVTPLIIYEETTDIWINIHDIFHVFPQSHEEEIEFIFASECKTGFRHLY
KITSILKESKYKRSSGGLPAPSDFKCPIKEEIAITSGEWEVLGRHGSNIQVDEVRRLVYF
EGTKDSPLEHHLYVVSYVNPGEVTRLTDRGYSHSCCISQHCDFFISKYSNQKNPHCVSLY
KLSSPEDDPTCKTKEFWATILDSAGPLPDYTPPEIFSFESTTGFTLYGMLYKPHDLQPGK
KYPTVLFIYGGPQVQLVNNRFKGVKYFRLNTLASLGYVVVVIDNRGSCHRGLKFEGAFKY
KMGQIEIDDQVEGLQYLASRYDFIDLDRVGIHGWSYGGYLSLMALMQRSDIFRVAIAGAP
VTLWIFYDTGYTERYMGHPDQNEQGYYLGSVAMQAEKFPSEPNRLLLLHGFLDENVHFAH
TSILLSFLVRAGKPYDLQIYPQERHSIRVPESGEHYELHLLHYLQENLGSRIAALKVI
|
|
|
|---|
| BDBM50214710 |
|---|
| n/a |
|---|
| Name | BDBM50214710 |
|---|
| Synonyms: | 2-(adamantan-1-ylamino)-1-[(R)-4-(5-methyl-[1,3,4]oxadiazole-2-carbonyl)-thiazolidin-3-yl]-ethanone | CHEMBL232111 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H26N4O3S |
|---|
| Mol. Mass. | 390.5 |
|---|
| SMILES | Cc1nnc(o1)C(=O)[C@@H]1CSCN1C(=O)CNC12CC3CC(CC(C3)C1)C2 |TLB:16:17:20:24.23.22,THB:18:19:22:26.17.25,18:17:20.19.24:22,25:17:20:24.23.22,25:23:20:26.18.17| |
|---|
| Structure | 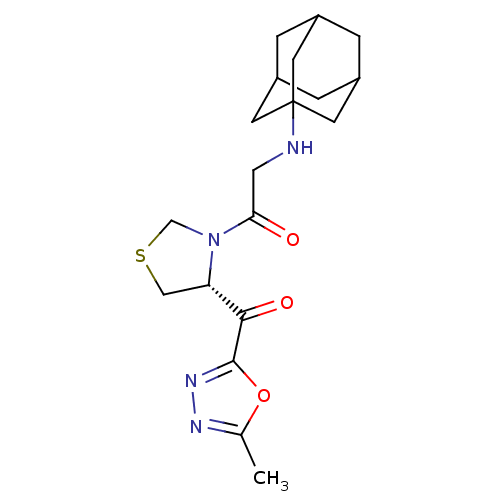
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Koo, KD; Kim, MJ; Kim, S; Kim, KH; Hong, SY; Hur, GC; Yim, HJ; Kim, GT; Han, HO; Kwon, OH; Kwon, TS; Koh, JS; Lee, CS Synthesis, SAR, and X-ray structure of novel potent DPPIV inhibitors: oxadiazolyl ketones. Bioorg Med Chem Lett17:4167-72 (2007) [PubMed] Article
Koo, KD; Kim, MJ; Kim, S; Kim, KH; Hong, SY; Hur, GC; Yim, HJ; Kim, GT; Han, HO; Kwon, OH; Kwon, TS; Koh, JS; Lee, CS Synthesis, SAR, and X-ray structure of novel potent DPPIV inhibitors: oxadiazolyl ketones. Bioorg Med Chem Lett17:4167-72 (2007) [PubMed] Article