 Found 944 hits with Last Name = 'lee' and Initial = 'cs'
Found 944 hits with Last Name = 'lee' and Initial = 'cs' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284986
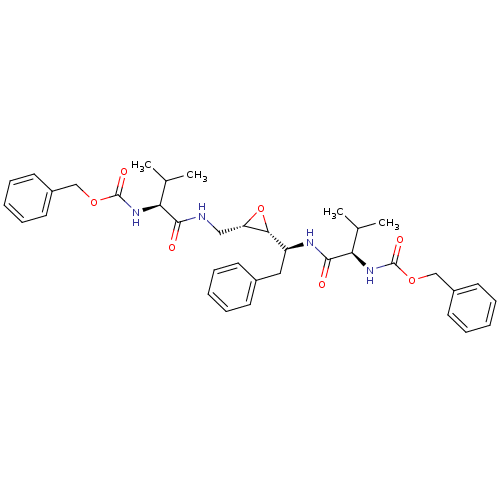
(CHEMBL55197 | [(R)-1-((S)-1-{(2R,3S)-3-[((S)-2-Ben...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)[C@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C37H46N4O7/c1-24(2)31(40-36(44)46-22-27-16-10-6-11-17-27)34(42)38-21-30-33(48-30)29(20-26-14-8-5-9-15-26)39-35(43)32(25(3)4)41-37(45)47-23-28-18-12-7-13-19-28/h5-19,24-25,29-33H,20-23H2,1-4H3,(H,38,42)(H,39,43)(H,40,44)(H,41,45)/t29-,30-,31-,32+,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition activity against HIV-1 protease |
Bioorg Med Chem Lett 5: 1843-1848 (1995)
Article DOI: 10.1016/0960-894X(95)00306-E
BindingDB Entry DOI: 10.7270/Q2KW5G0J |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288943
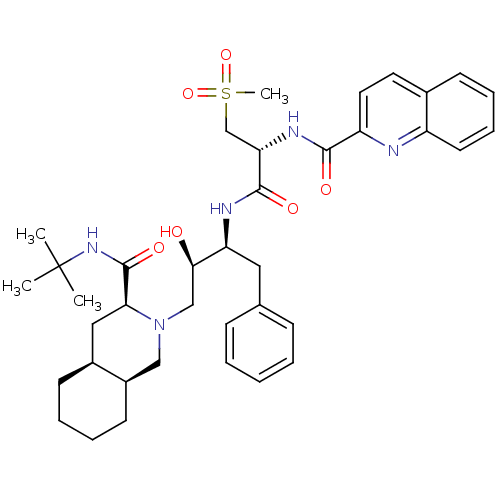
(CHEMBL154519 | Quinoline-2-carboxylic acid {(R)-1-...)Show SMILES CC(C)(C)NC(=O)[C@@H]1C[C@@H]2CCCC[C@@H]2CN1C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CS(C)(=O)=O)NC(=O)c1ccc2ccccc2n1 Show InChI InChI=1S/C38H51N5O6S/c1-38(2,3)42-37(47)33-21-27-15-8-9-16-28(27)22-43(33)23-34(44)31(20-25-12-6-5-7-13-25)40-36(46)32(24-50(4,48)49)41-35(45)30-19-18-26-14-10-11-17-29(26)39-30/h5-7,10-14,17-19,27-28,31-34,44H,8-9,15-16,20-24H2,1-4H3,(H,40,46)(H,41,45)(H,42,47)/t27-,28+,31-,32-,33-,34+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against P2 site in HIV protease. |
Bioorg Med Chem Lett 6: 585-588 (1996)
Article DOI: 10.1016/0960-894X(96)00086-8
BindingDB Entry DOI: 10.7270/Q2KH0N9Z |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288941
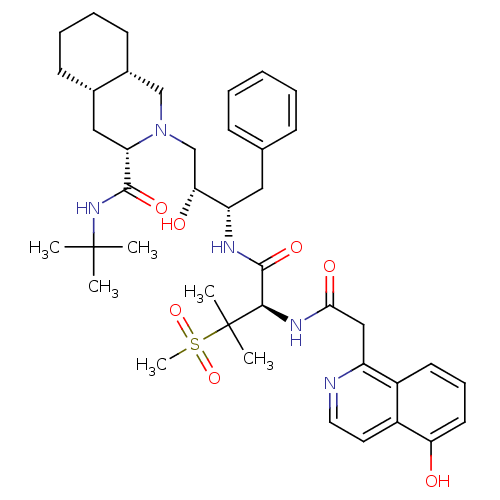
((3S,4aS,8aS)-2-((2R,3S)-2-Hydroxy-3-{(R)-2-[2-(5-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1C[C@@H]2CCCC[C@@H]2CN1C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)Cc1nccc2c(O)cccc12)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C41H57N5O7S/c1-40(2,3)45-38(50)33-22-27-15-10-11-16-28(27)24-46(33)25-35(48)32(21-26-13-8-7-9-14-26)43-39(51)37(41(4,5)54(6,52)53)44-36(49)23-31-29-17-12-18-34(47)30(29)19-20-42-31/h7-9,12-14,17-20,27-28,32-33,35,37,47-48H,10-11,15-16,21-25H2,1-6H3,(H,43,51)(H,44,49)(H,45,50)/t27-,28+,32-,33-,35+,37+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 0.151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against P2 site in HIV protease. |
Bioorg Med Chem Lett 6: 585-588 (1996)
Article DOI: 10.1016/0960-894X(96)00086-8
BindingDB Entry DOI: 10.7270/Q2KH0N9Z |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288942
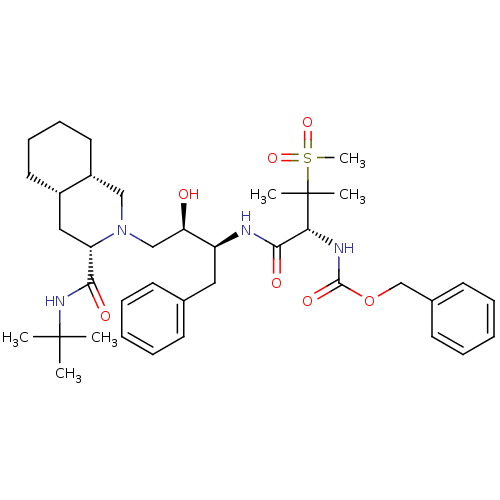
(CHEMBL154692 | {(R)-1-[(1S,2R)-1-Benzyl-3-((3S,4aS...)Show SMILES CC(C)(C)NC(=O)[C@@H]1C[C@@H]2CCCC[C@@H]2CN1C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C38H56N4O7S/c1-37(2,3)41-34(44)31-22-28-19-13-14-20-29(28)23-42(31)24-32(43)30(21-26-15-9-7-10-16-26)39-35(45)33(38(4,5)50(6,47)48)40-36(46)49-25-27-17-11-8-12-18-27/h7-12,15-18,28-33,43H,13-14,19-25H2,1-6H3,(H,39,45)(H,40,46)(H,41,44)/t28-,29+,30-,31-,32+,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against P2 site in HIV protease. |
Bioorg Med Chem Lett 6: 585-588 (1996)
Article DOI: 10.1016/0960-894X(96)00086-8
BindingDB Entry DOI: 10.7270/Q2KH0N9Z |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288939
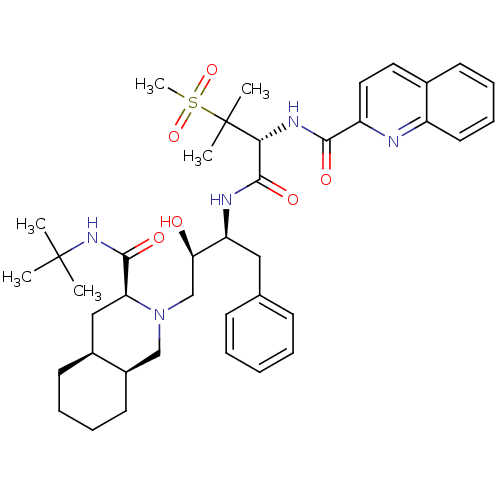
(CHEMBL345187 | Quinoline-2-carboxylic acid {(R)-1-...)Show SMILES CC(C)(C)NC(=O)[C@@H]1C[C@@H]2CCCC[C@@H]2CN1C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccc2ccccc2n1)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C40H55N5O6S/c1-39(2,3)44-37(48)33-23-28-17-10-11-18-29(28)24-45(33)25-34(46)32(22-26-14-8-7-9-15-26)42-38(49)35(40(4,5)52(6,50)51)43-36(47)31-21-20-27-16-12-13-19-30(27)41-31/h7-9,12-16,19-21,28-29,32-35,46H,10-11,17-18,22-25H2,1-6H3,(H,42,49)(H,43,47)(H,44,48)/t28-,29+,32-,33-,34+,35+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against P2 site in HIV protease. |
Bioorg Med Chem Lett 6: 585-588 (1996)
Article DOI: 10.1016/0960-894X(96)00086-8
BindingDB Entry DOI: 10.7270/Q2KH0N9Z |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM519

((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 0.452 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against P2 site in HIV protease. |
Bioorg Med Chem Lett 6: 585-588 (1996)
Article DOI: 10.1016/0960-894X(96)00086-8
BindingDB Entry DOI: 10.7270/Q2KH0N9Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM9294

((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...)Show SMILES CC(C)[C@H](NC(=O)c1ccc2ccccc2n1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C39H53N5O4/c1-25(2)35(42-36(46)31-20-19-27-15-11-12-18-30(27)40-31)38(48)41-32(21-26-13-7-6-8-14-26)34(45)24-44-23-29-17-10-9-16-28(29)22-33(44)37(47)43-39(3,4)5/h6-8,11-15,18-20,25,28-29,32-35,45H,9-10,16-17,21-24H2,1-5H3,(H,41,48)(H,42,46)(H,43,47)/t28-,29+,32-,33-,34+,35-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against P2 site in HIV protease. |
Bioorg Med Chem Lett 6: 585-588 (1996)
Article DOI: 10.1016/0960-894X(96)00086-8
BindingDB Entry DOI: 10.7270/Q2KH0N9Z |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288947

(CHEMBL158033 | N*1*-((S)-1-{(2R,3S)-3-[(1-Isopropy...)Show SMILES CC(C)C(NC(=O)C[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)C(CC(N)=O)NC(=O)c1ccc2ccccc2n1)C(C)C Show InChI InChI=1S/C33H41N5O5/c1-19(2)30(20(3)4)38-29(40)18-27-31(43-27)25(16-21-10-6-5-7-11-21)36-33(42)26(17-28(34)39)37-32(41)24-15-14-22-12-8-9-13-23(22)35-24/h5-15,19-20,25-27,30-31H,16-18H2,1-4H3,(H2,34,39)(H,36,42)(H,37,41)(H,38,40)/t25-,26?,27-,31+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV protease was determined |
Bioorg Med Chem Lett 6: 589-594 (1996)
Article DOI: 10.1016/0960-894X(96)00087-X
BindingDB Entry DOI: 10.7270/Q2FQ9WM4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50009242
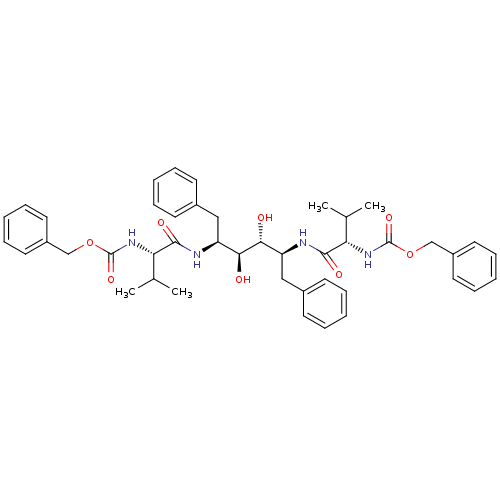
(CHEMBL48565 | {(S)-1-[(1S,2S,3R,4S)-1-Benzyl-4-((S...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C44H54N4O8/c1-29(2)37(47-43(53)55-27-33-21-13-7-14-22-33)41(51)45-35(25-31-17-9-5-10-18-31)39(49)40(50)36(26-32-19-11-6-12-20-32)46-42(52)38(30(3)4)48-44(54)56-28-34-23-15-8-16-24-34/h5-24,29-30,35-40,49-50H,25-28H2,1-4H3,(H,45,51)(H,46,52)(H,47,53)(H,48,54)/t35-,36-,37-,38-,39-,40+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition activity against HIV-1 protease |
Bioorg Med Chem Lett 5: 1843-1848 (1995)
Article DOI: 10.1016/0960-894X(95)00306-E
BindingDB Entry DOI: 10.7270/Q2KW5G0J |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM197
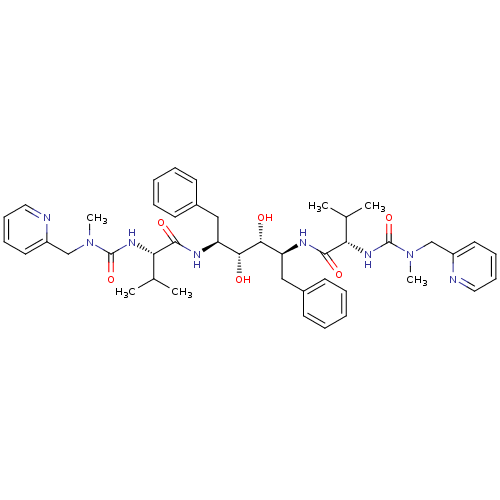
((2S)-N-[(2S,3R,4R,5S)-3,4-dihydroxy-5-[(2S)-3-meth...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1ccccn1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)N(C)Cc1ccccn1)C(C)C |r| Show InChI InChI=1S/C44H58N8O6/c1-29(2)37(49-43(57)51(5)27-33-21-13-15-23-45-33)41(55)47-35(25-31-17-9-7-10-18-31)39(53)40(54)36(26-32-19-11-8-12-20-32)48-42(56)38(30(3)4)50-44(58)52(6)28-34-22-14-16-24-46-34/h7-24,29-30,35-40,53-54H,25-28H2,1-6H3,(H,47,55)(H,48,56)(H,49,57)(H,50,58)/t35-,36-,37-,38-,39+,40+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition activity against HIV-1 protease |
Bioorg Med Chem Lett 5: 1843-1848 (1995)
Article DOI: 10.1016/0960-894X(95)00306-E
BindingDB Entry DOI: 10.7270/Q2KW5G0J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187167
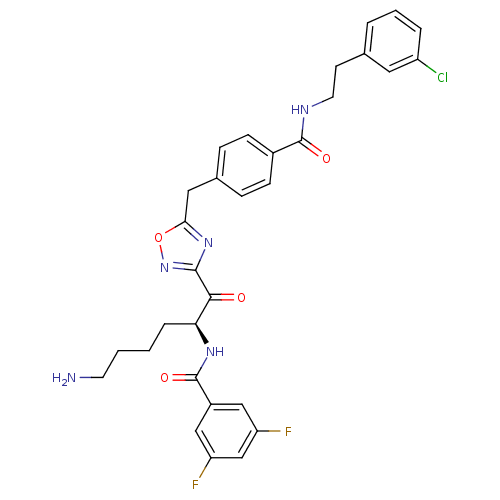
(CHEMBL211357 | N-[(S)-5-amino-1-(5-{4-[2-(3-chloro...)Show SMILES NCCCC[C@H](NC(=O)c1cc(F)cc(F)c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H30ClF2N5O4/c32-23-5-3-4-19(14-23)11-13-36-30(41)21-9-7-20(8-10-21)15-27-38-29(39-43-27)28(40)26(6-1-2-12-35)37-31(42)22-16-24(33)18-25(34)17-22/h3-5,7-10,14,16-18,26H,1-2,6,11-13,15,35H2,(H,36,41)(H,37,42)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187166
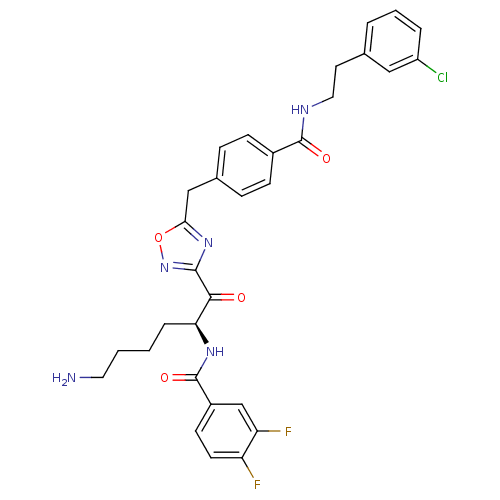
(CHEMBL380293 | N-[(S)-5-amino-1-(5-{4-[2-(3-chloro...)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H30ClF2N5O4/c32-23-5-3-4-19(16-23)13-15-36-30(41)21-9-7-20(8-10-21)17-27-38-29(39-43-27)28(40)26(6-1-2-14-35)37-31(42)22-11-12-24(33)25(34)18-22/h3-5,7-12,16,18,26H,1-2,6,13-15,17,35H2,(H,36,41)(H,37,42)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187177
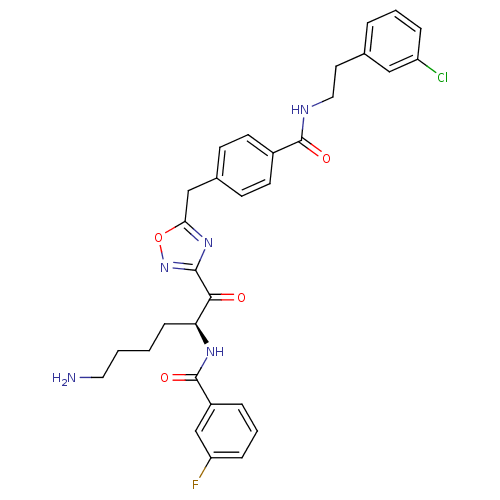
(CHEMBL212774 | N-[(2S)-6-amino-1-{5-[(4-{[2-(3-chl...)Show SMILES NCCCC[C@H](NC(=O)c1cccc(F)c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H31ClFN5O4/c32-24-7-3-5-20(17-24)14-16-35-30(40)22-12-10-21(11-13-22)18-27-37-29(38-42-27)28(39)26(9-1-2-15-34)36-31(41)23-6-4-8-25(33)19-23/h3-8,10-13,17,19,26H,1-2,9,14-16,18,34H2,(H,35,40)(H,36,41)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187163
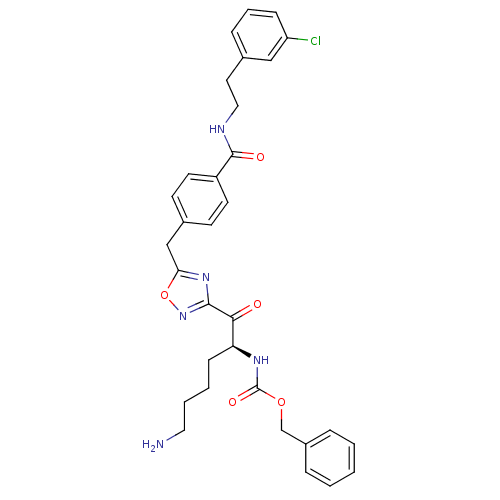
((S)-benzyl 1-(5-(4-((3-chlorophenethyl)carbamoyl)b...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C32H34ClN5O5/c33-26-10-6-9-22(19-26)16-18-35-31(40)25-14-12-23(13-15-25)20-28-37-30(38-43-28)29(39)27(11-4-5-17-34)36-32(41)42-21-24-7-2-1-3-8-24/h1-3,6-10,12-15,19,27H,4-5,11,16-18,20-21,34H2,(H,35,40)(H,36,41)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187160

(CHEMBL213928 | N-[(2S)-6-amino-1-{5-[(4-{[2-(3-chl...)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)cc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H31ClFN5O4/c32-24-5-3-4-20(18-24)15-17-35-30(40)22-9-7-21(8-10-22)19-27-37-29(38-42-27)28(39)26(6-1-2-16-34)36-31(41)23-11-13-25(33)14-12-23/h3-5,7-14,18,26H,1-2,6,15-17,19,34H2,(H,35,40)(H,36,41)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187176
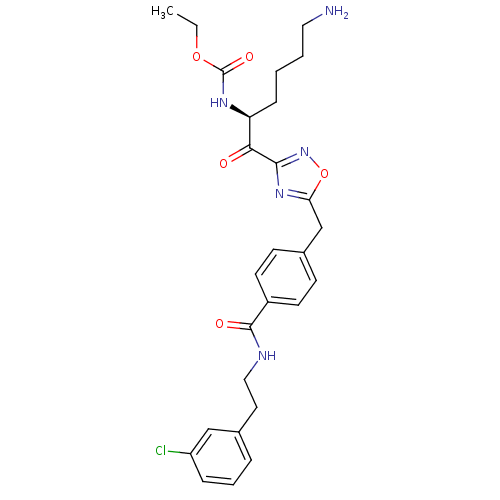
((S)-ethyl 1-(5-(4-((3-chlorophenethyl)carbamoyl)be...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C27H32ClN5O5/c1-2-37-27(36)31-22(8-3-4-14-29)24(34)25-32-23(38-33-25)17-19-9-11-20(12-10-19)26(35)30-15-13-18-6-5-7-21(28)16-18/h5-7,9-12,16,22H,2-4,8,13-15,17,29H2,1H3,(H,30,35)(H,31,36)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187168
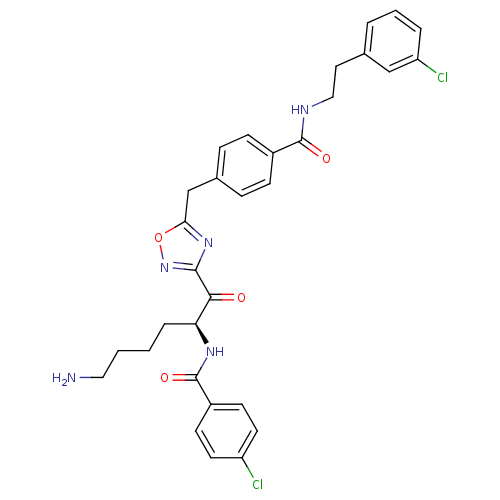
(CHEMBL377656 | N-[(2S)-6-amino-1-{5-[(4-{[2-(3-chl...)Show SMILES NCCCC[C@H](NC(=O)c1ccc(Cl)cc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H31Cl2N5O4/c32-24-13-11-23(12-14-24)31(41)36-26(6-1-2-16-34)28(39)29-37-27(42-38-29)19-21-7-9-22(10-8-21)30(40)35-17-15-20-4-3-5-25(33)18-20/h3-5,7-14,18,26H,1-2,6,15-17,19,34H2,(H,35,40)(H,36,41)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187162
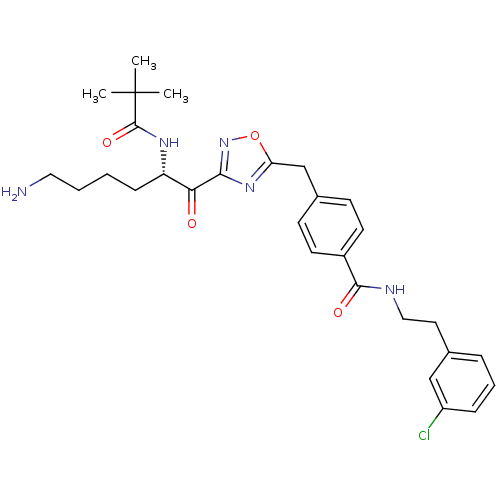
((S)-N-(3-chlorophenethyl)-4-((3-(6-amino-2-pivalam...)Show SMILES CC(C)(C)C(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C29H36ClN5O4/c1-29(2,3)28(38)33-23(9-4-5-15-31)25(36)26-34-24(39-35-26)18-20-10-12-21(13-11-20)27(37)32-16-14-19-7-6-8-22(30)17-19/h6-8,10-13,17,23H,4-5,9,14-16,18,31H2,1-3H3,(H,32,37)(H,33,38)/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187182

((S)-N-(1-(5-(4-((3-chlorophenethyl)carbamoyl)benzy...)Show SMILES NCCCC[C@H](NC(=O)c1ccc2OCOc2c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C32H32ClN5O6/c33-24-5-3-4-20(16-24)13-15-35-31(40)22-9-7-21(8-10-22)17-28-37-30(38-44-28)29(39)25(6-1-2-14-34)36-32(41)23-11-12-26-27(18-23)43-19-42-26/h3-5,7-12,16,18,25H,1-2,6,13-15,17,19,34H2,(H,35,40)(H,36,41)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187164
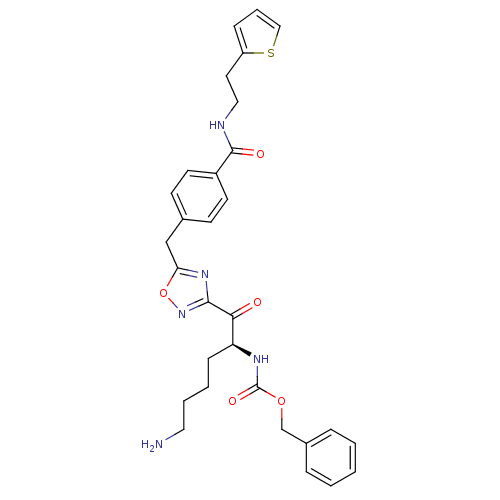
((S)-benzyl 1-(5-(4-((2-(thiophen-2-yl)ethyl)carbam...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccs2)n1 Show InChI InChI=1S/C30H33N5O5S/c31-16-5-4-10-25(33-30(38)39-20-22-7-2-1-3-8-22)27(36)28-34-26(40-35-28)19-21-11-13-23(14-12-21)29(37)32-17-15-24-9-6-18-41-24/h1-3,6-9,11-14,18,25H,4-5,10,15-17,19-20,31H2,(H,32,37)(H,33,38)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288940

(CHEMBL154416 | Quinoline-2-carboxylic acid {(R)-1-...)Show SMILES CSC(C)(C)[C@H](NC(=O)c1ccc2ccccc2n1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C Show InChI InChI=1S/C40H55N5O4S/c1-39(2,3)44-37(48)33-23-28-17-10-11-18-29(28)24-45(33)25-34(46)32(22-26-14-8-7-9-15-26)42-38(49)35(40(4,5)50-6)43-36(47)31-21-20-27-16-12-13-19-30(27)41-31/h7-9,12-16,19-21,28-29,32-35,46H,10-11,17-18,22-25H2,1-6H3,(H,42,49)(H,43,47)(H,44,48)/t28-,29+,32-,33-,34+,35+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against P2 site in HIV protease. |
Bioorg Med Chem Lett 6: 585-588 (1996)
Article DOI: 10.1016/0960-894X(96)00086-8
BindingDB Entry DOI: 10.7270/Q2KH0N9Z |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM14312
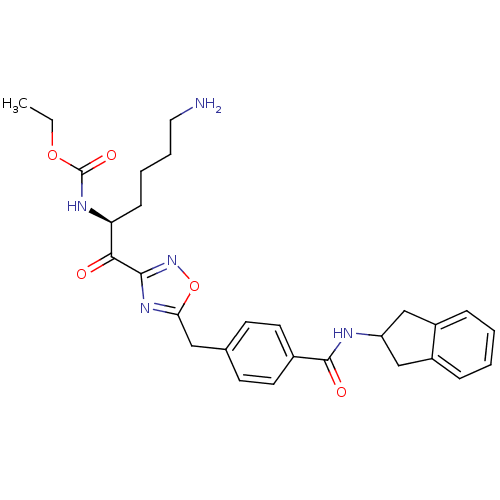
(CHEMBL214368 | CRA23 | ethyl N-[(2S)-6-amino-1-(5-...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(cc2)C(=O)NC2Cc3ccccc3C2)n1 |r| Show InChI InChI=1S/C28H33N5O5/c1-2-37-28(36)31-23(9-5-6-14-29)25(34)26-32-24(38-33-26)15-18-10-12-19(13-11-18)27(35)30-22-16-20-7-3-4-8-21(20)17-22/h3-4,7-8,10-13,22-23H,2,5-6,9,14-17,29H2,1H3,(H,30,35)(H,31,36)/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187169
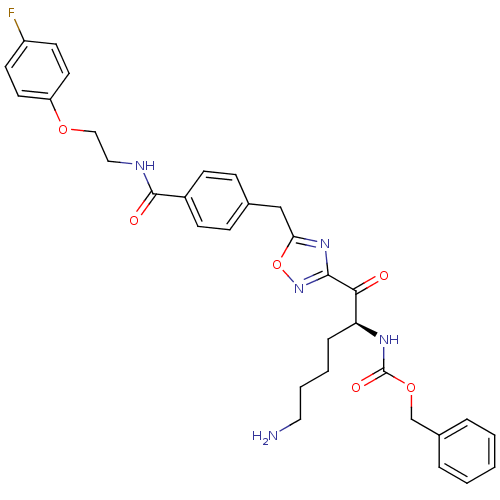
((S)-benzyl 1-(5-(4-((2-(4-fluorophenoxy)ethyl)carb...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCOc2ccc(F)cc2)n1 Show InChI InChI=1S/C32H34FN5O6/c33-25-13-15-26(16-14-25)42-19-18-35-31(40)24-11-9-22(10-12-24)20-28-37-30(38-44-28)29(39)27(8-4-5-17-34)36-32(41)43-21-23-6-2-1-3-7-23/h1-3,6-7,9-16,27H,4-5,8,17-21,34H2,(H,35,40)(H,36,41)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM14312

(CHEMBL214368 | CRA23 | ethyl N-[(2S)-6-amino-1-(5-...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(cc2)C(=O)NC2Cc3ccccc3C2)n1 |r| Show InChI InChI=1S/C28H33N5O5/c1-2-37-28(36)31-23(9-5-6-14-29)25(34)26-32-24(38-33-26)15-18-10-12-19(13-11-18)27(35)30-22-16-20-7-3-4-8-21(20)17-22/h3-4,7-8,10-13,22-23H,2,5-6,9,14-17,29H2,1H3,(H,30,35)(H,31,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Biochemistry 45: 5964-73 (2006)
Article DOI: 10.1021/bi060173m
BindingDB Entry DOI: 10.7270/Q2W09450 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187179
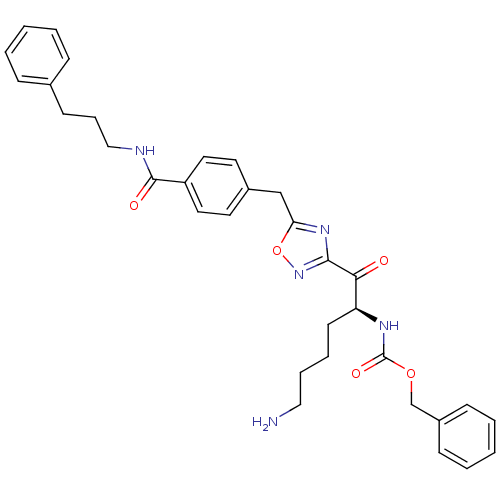
((S)-benzyl 1-(5-(4-((3-phenylpropyl)carbamoyl)benz...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCCc2ccccc2)n1 Show InChI InChI=1S/C33H37N5O5/c34-20-8-7-15-28(36-33(41)42-23-26-12-5-2-6-13-26)30(39)31-37-29(43-38-31)22-25-16-18-27(19-17-25)32(40)35-21-9-14-24-10-3-1-4-11-24/h1-6,10-13,16-19,28H,7-9,14-15,20-23,34H2,(H,35,40)(H,36,41)/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187154
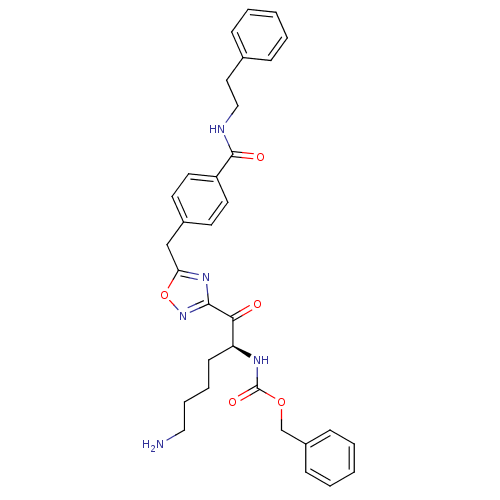
((S)-benzyl 1-(5-(4-(phenethylcarbamoyl)benzyl)-1,2...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2ccccc2)n1 Show InChI InChI=1S/C32H35N5O5/c33-19-8-7-13-27(35-32(40)41-22-25-11-5-2-6-12-25)29(38)30-36-28(42-37-30)21-24-14-16-26(17-15-24)31(39)34-20-18-23-9-3-1-4-10-23/h1-6,9-12,14-17,27H,7-8,13,18-22,33H2,(H,34,39)(H,35,40)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187157

((S)-benzyl 1-(5-(4-((3,5-difluorophenethyl)carbamo...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cc(F)cc(F)c2)n1 Show InChI InChI=1S/C32H33F2N5O5/c33-25-16-23(17-26(34)19-25)13-15-36-31(41)24-11-9-21(10-12-24)18-28-38-30(39-44-28)29(40)27(8-4-5-14-35)37-32(42)43-20-22-6-2-1-3-7-22/h1-3,6-7,9-12,16-17,19,27H,4-5,8,13-15,18,20,35H2,(H,36,41)(H,37,42)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187165
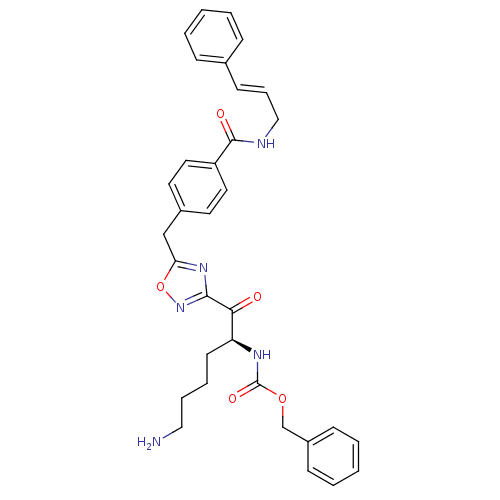
((S)-benzyl 1-(5-(4-(cinnamylcarbamoyl)benzyl)-1,2,...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NC\C=C\c2ccccc2)n1 Show InChI InChI=1S/C33H35N5O5/c34-20-8-7-15-28(36-33(41)42-23-26-12-5-2-6-13-26)30(39)31-37-29(43-38-31)22-25-16-18-27(19-17-25)32(40)35-21-9-14-24-10-3-1-4-11-24/h1-6,9-14,16-19,28H,7-8,15,20-23,34H2,(H,35,40)(H,36,41)/b14-9+/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187158
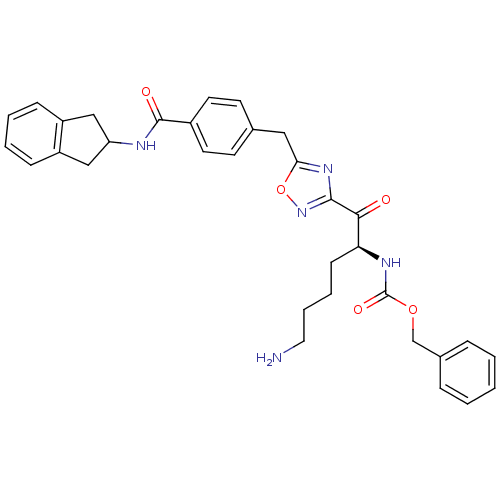
((S)-benzyl 1-(5-(4-((2,3-dihydro-1H-inden-2-yl)car...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NC2Cc3ccccc3C2)n1 Show InChI InChI=1S/C33H35N5O5/c34-17-7-6-12-28(36-33(41)42-21-23-8-2-1-3-9-23)30(39)31-37-29(43-38-31)18-22-13-15-24(16-14-22)32(40)35-27-19-25-10-4-5-11-26(25)20-27/h1-5,8-11,13-16,27-28H,6-7,12,17-21,34H2,(H,35,40)(H,36,41)/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM14311
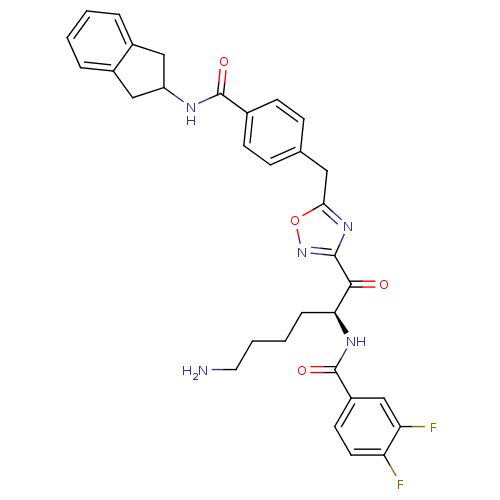
(CRA22 | N-[(2S)-6-amino-1-(5-{[4-(2,3-dihydro-1H-i...)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NC2Cc3ccccc3C2)n1 |r| Show InChI InChI=1S/C32H31F2N5O4/c33-25-13-12-23(18-26(25)34)32(42)37-27(7-3-4-14-35)29(40)30-38-28(43-39-30)15-19-8-10-20(11-9-19)31(41)36-24-16-21-5-1-2-6-22(21)17-24/h1-2,5-6,8-13,18,24,27H,3-4,7,14-17,35H2,(H,36,41)(H,37,42)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Biochemistry 45: 5964-73 (2006)
Article DOI: 10.1021/bi060173m
BindingDB Entry DOI: 10.7270/Q2W09450 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284988
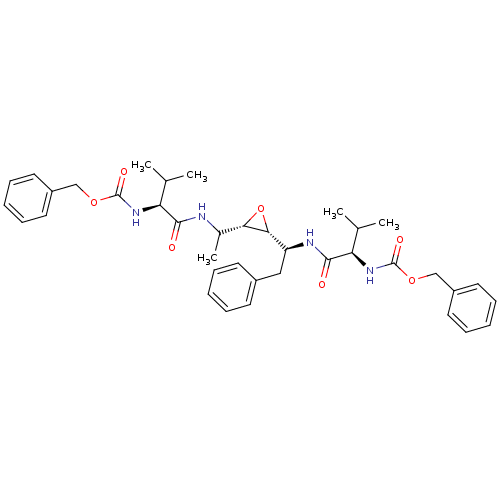
(CHEMBL52809 | [(S)-1-(1-{(2S,3R)-3-[(S)-1-((R)-2-B...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(C)[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)[C@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C38H48N4O7/c1-24(2)31(41-37(45)47-22-28-17-11-7-12-18-28)35(43)39-26(5)33-34(49-33)30(21-27-15-9-6-10-16-27)40-36(44)32(25(3)4)42-38(46)48-23-29-19-13-8-14-20-29/h6-20,24-26,30-34H,21-23H2,1-5H3,(H,39,43)(H,40,44)(H,41,45)(H,42,46)/t26?,30-,31-,32+,33-,34+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition activity against HIV-1 protease |
Bioorg Med Chem Lett 5: 1843-1848 (1995)
Article DOI: 10.1016/0960-894X(95)00306-E
BindingDB Entry DOI: 10.7270/Q2KW5G0J |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187800
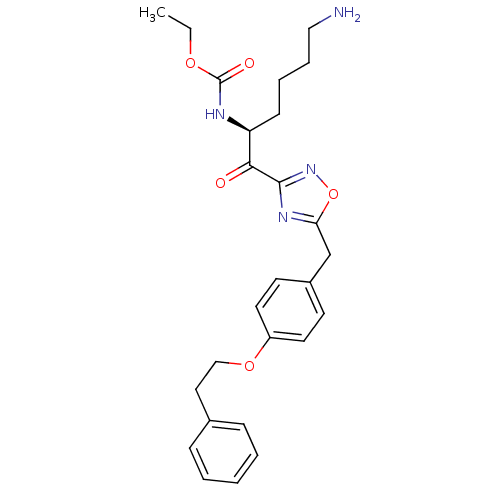
((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to human tryptase beta2 |
Bioorg Med Chem Lett 16: 4085-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.088
BindingDB Entry DOI: 10.7270/Q2X929W7 |
More data for this
Ligand-Target Pair | |
Tryptase
(Rattus norvegicus) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to rat tryptase |
Bioorg Med Chem Lett 16: 4085-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.088
BindingDB Entry DOI: 10.7270/Q2X929W7 |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187172
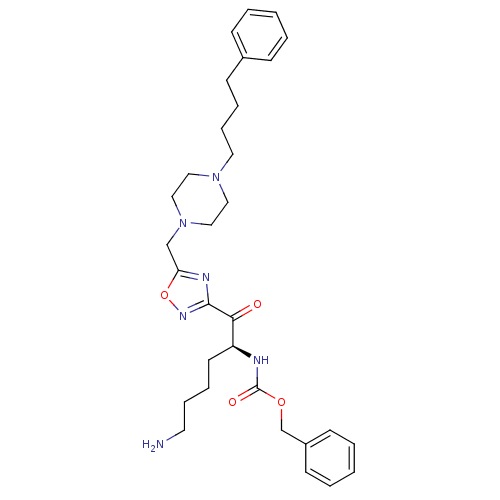
((S)-benzyl 6-amino-1-oxo-1-(5-((4-(4-phenylbutyl)p...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(CN2CCN(CCCCc3ccccc3)CC2)n1 Show InChI InChI=1S/C31H42N6O4/c32-17-9-7-16-27(33-31(39)40-24-26-14-5-2-6-15-26)29(38)30-34-28(41-35-30)23-37-21-19-36(20-22-37)18-10-8-13-25-11-3-1-4-12-25/h1-6,11-12,14-15,27H,7-10,13,16-24,32H2,(H,33,39)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM14316
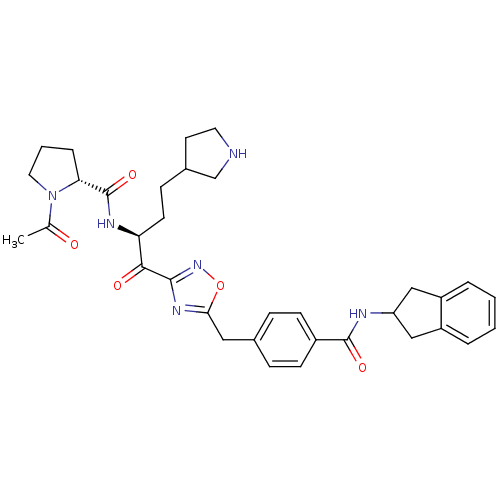
((2R)-N-[(2S)-1-(5-{[4-(2,3-dihydro-1H-inden-2-ylca...)Show SMILES CC(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCC1CCNC1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NC2Cc3ccccc3C2)n1 |r| Show InChI InChI=1S/C34H40N6O5/c1-21(41)40-16-4-7-29(40)34(44)37-28(13-10-23-14-15-35-20-23)31(42)32-38-30(45-39-32)17-22-8-11-24(12-9-22)33(43)36-27-18-25-5-2-3-6-26(25)19-27/h2-3,5-6,8-9,11-12,23,27-29,35H,4,7,10,13-20H2,1H3,(H,36,43)(H,37,44)/t23?,28-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Biochemistry 45: 5964-73 (2006)
Article DOI: 10.1021/bi060173m
BindingDB Entry DOI: 10.7270/Q2W09450 |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187178
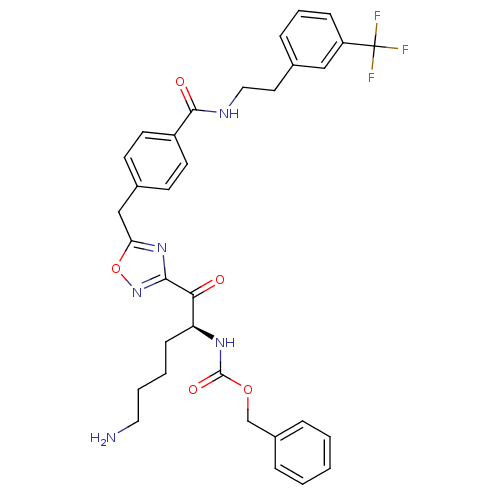
((S)-benzyl 1-(5-(4-((3-(trifluoromethyl)phenethyl)...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(c2)C(F)(F)F)n1 Show InChI InChI=1S/C33H34F3N5O5/c34-33(35,36)26-10-6-9-22(19-26)16-18-38-31(43)25-14-12-23(13-15-25)20-28-40-30(41-46-28)29(42)27(11-4-5-17-37)39-32(44)45-21-24-7-2-1-3-8-24/h1-3,6-10,12-15,19,27H,4-5,11,16-18,20-21,37H2,(H,38,43)(H,39,44)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187151
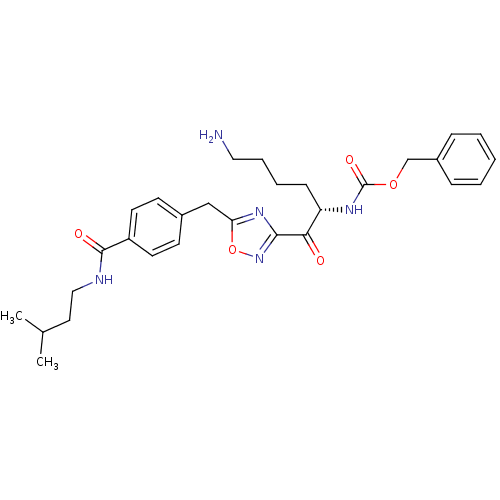
((S)-benzyl 1-(5-(4-(isopentylcarbamoyl)benzyl)-1,2...)Show SMILES CC(C)CCNC(=O)c1ccc(Cc2nc(no2)C(=O)[C@H](CCCCN)NC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C29H37N5O5/c1-20(2)15-17-31-28(36)23-13-11-21(12-14-23)18-25-33-27(34-39-25)26(35)24(10-6-7-16-30)32-29(37)38-19-22-8-4-3-5-9-22/h3-5,8-9,11-14,20,24H,6-7,10,15-19,30H2,1-2H3,(H,31,36)(H,32,37)/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187175
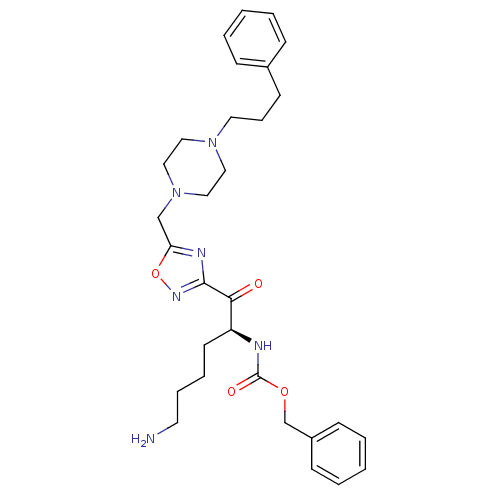
((S)-benzyl 6-amino-1-oxo-1-(5-((4-(3-phenylpropyl)...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(CN2CCN(CCCc3ccccc3)CC2)n1 Show InChI InChI=1S/C30H40N6O4/c31-16-8-7-15-26(32-30(38)39-23-25-12-5-2-6-13-25)28(37)29-33-27(40-34-29)22-36-20-18-35(19-21-36)17-9-14-24-10-3-1-4-11-24/h1-6,10-13,26H,7-9,14-23,31H2,(H,32,38)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187171

((S)-benzyl 1-(5-(4-((naphthalen-2-ylmethyl)carbamo...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCc2ccc3ccccc3c2)n1 Show InChI InChI=1S/C35H35N5O5/c36-19-7-6-12-30(38-35(43)44-23-25-8-2-1-3-9-25)32(41)33-39-31(45-40-33)21-24-13-17-28(18-14-24)34(42)37-22-26-15-16-27-10-4-5-11-29(27)20-26/h1-5,8-11,13-18,20,30H,6-7,12,19,21-23,36H2,(H,37,42)(H,38,43)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284989
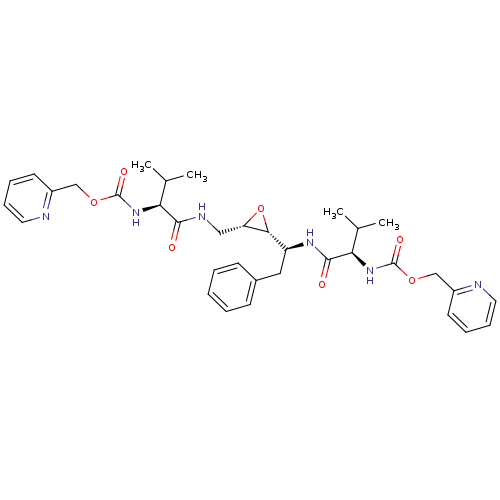
(CHEMBL300705 | {(R)-2-Methyl-1-[(S)-1-((2R,3S)-3-{...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccn1)C(=O)NC[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)[C@H](NC(=O)OCc1ccccn1)C(C)C Show InChI InChI=1S/C35H44N6O7/c1-22(2)29(40-34(44)46-20-25-14-8-10-16-36-25)32(42)38-19-28-31(48-28)27(18-24-12-6-5-7-13-24)39-33(43)30(23(3)4)41-35(45)47-21-26-15-9-11-17-37-26/h5-17,22-23,27-31H,18-21H2,1-4H3,(H,38,42)(H,39,43)(H,40,44)(H,41,45)/t27-,28-,29-,30+,31+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition activity against HIV-1 protease |
Bioorg Med Chem Lett 5: 1843-1848 (1995)
Article DOI: 10.1016/0960-894X(95)00306-E
BindingDB Entry DOI: 10.7270/Q2KW5G0J |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187152
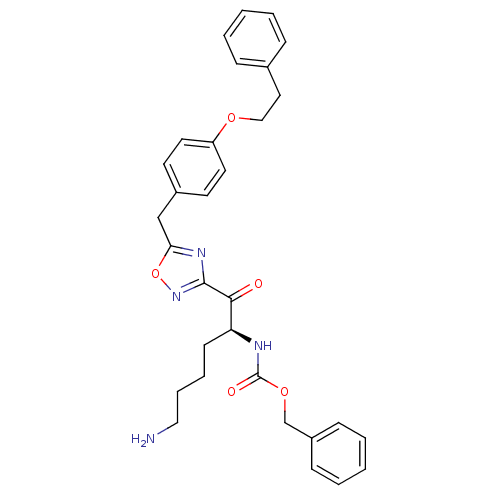
((S)-benzyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiaz...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C31H34N4O5/c32-19-8-7-13-27(33-31(37)39-22-25-11-5-2-6-12-25)29(36)30-34-28(40-35-30)21-24-14-16-26(17-15-24)38-20-18-23-9-3-1-4-10-23/h1-6,9-12,14-17,27H,7-8,13,18-22,32H2,(H,33,37)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187173
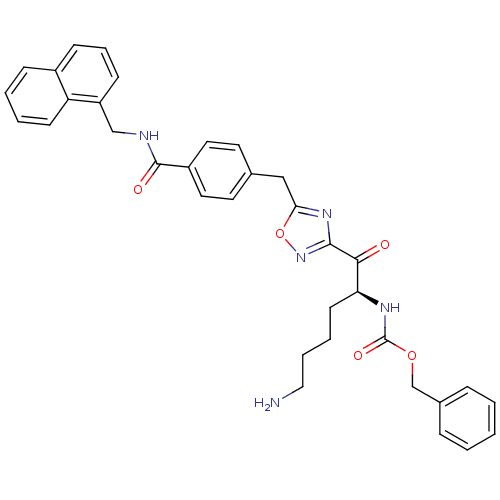
((S)-benzyl 1-(5-(4-((naphthalen-1-ylmethyl)carbamo...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCc2cccc3ccccc23)n1 Show InChI InChI=1S/C35H35N5O5/c36-20-7-6-15-30(38-35(43)44-23-25-9-2-1-3-10-25)32(41)33-39-31(45-40-33)21-24-16-18-27(19-17-24)34(42)37-22-28-13-8-12-26-11-4-5-14-29(26)28/h1-5,8-14,16-19,30H,6-7,15,20-23,36H2,(H,37,42)(H,38,43)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187180
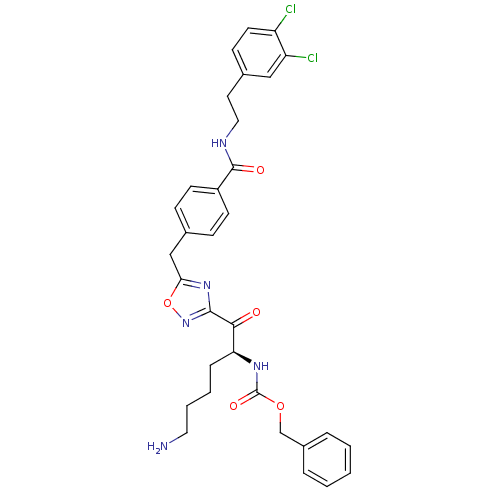
((S)-benzyl 1-(5-(4-((3,4-dichlorophenethyl)carbamo...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2ccc(Cl)c(Cl)c2)n1 Show InChI InChI=1S/C32H33Cl2N5O5/c33-25-14-11-22(18-26(25)34)15-17-36-31(41)24-12-9-21(10-13-24)19-28-38-30(39-44-28)29(40)27(8-4-5-16-35)37-32(42)43-20-23-6-2-1-3-7-23/h1-3,6-7,9-14,18,27H,4-5,8,15-17,19-20,35H2,(H,36,41)(H,37,42)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284984
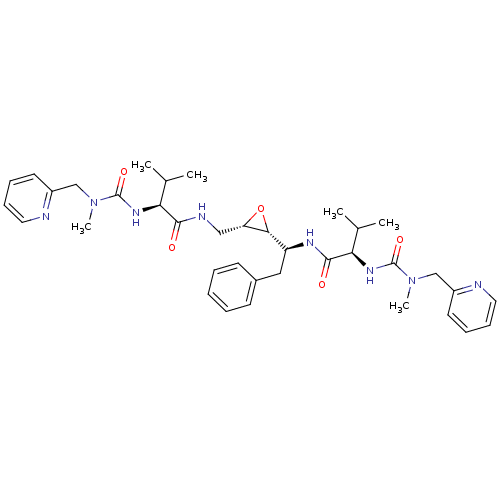
((R)-3-Methyl-N-[(S)-1-((2R,3S)-3-{[(S)-3-methyl-2-...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1ccccn1)C(=O)NC[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)[C@H](NC(=O)N(C)Cc1ccccn1)C(C)C Show InChI InChI=1S/C37H50N8O5/c1-24(2)31(42-36(48)44(5)22-27-16-10-12-18-38-27)34(46)40-21-30-33(50-30)29(20-26-14-8-7-9-15-26)41-35(47)32(25(3)4)43-37(49)45(6)23-28-17-11-13-19-39-28/h7-19,24-25,29-33H,20-23H2,1-6H3,(H,40,46)(H,41,47)(H,42,48)(H,43,49)/t29-,30-,31-,32+,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition activity against HIV-1 protease |
Bioorg Med Chem Lett 5: 1843-1848 (1995)
Article DOI: 10.1016/0960-894X(95)00306-E
BindingDB Entry DOI: 10.7270/Q2KW5G0J |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288946
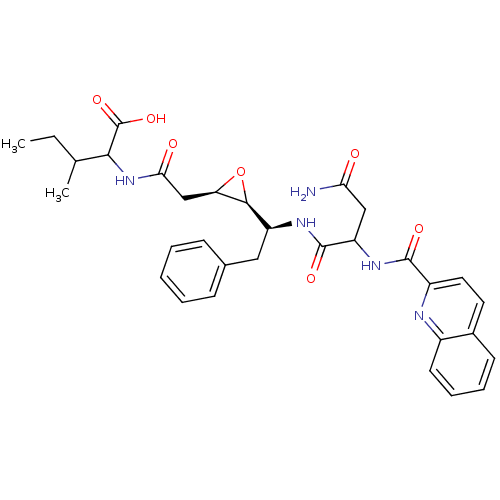
(2-{2-[(2R,3S)-3-((S)-1-{3-Carbamoyl-2-[(quinoline-...)Show SMILES CCC(C)C(NC(=O)C[C@H]1O[C@H]1[C@H](Cc1ccccc1)NC(=O)C(CC(N)=O)NC(=O)c1ccc2ccccc2n1)C(O)=O Show InChI InChI=1S/C32H37N5O7/c1-3-18(2)28(32(42)43)37-27(39)17-25-29(44-25)23(15-19-9-5-4-6-10-19)35-31(41)24(16-26(33)38)36-30(40)22-14-13-20-11-7-8-12-21(20)34-22/h4-14,18,23-25,28-29H,3,15-17H2,1-2H3,(H2,33,38)(H,35,41)(H,36,40)(H,37,39)(H,42,43)/t18?,23-,24?,25+,28?,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV protease was determined |
Bioorg Med Chem Lett 6: 589-594 (1996)
Article DOI: 10.1016/0960-894X(96)00087-X
BindingDB Entry DOI: 10.7270/Q2FQ9WM4 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM14317
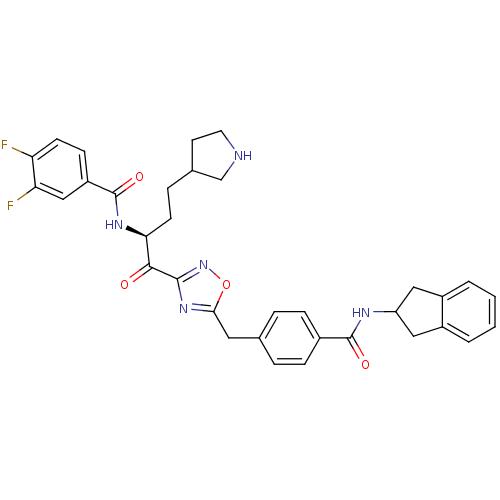
(CRA28 | N-[(2S)-1-(5-{[4-(2,3-dihydro-1H-inden-2-y...)Show SMILES Fc1ccc(cc1F)C(=O)N[C@@H](CCC1CCNC1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NC2Cc3ccccc3C2)n1 |r| Show InChI InChI=1S/C34H33F2N5O4/c35-27-11-10-25(18-28(27)36)34(44)39-29(12-7-21-13-14-37-19-21)31(42)32-40-30(45-41-32)15-20-5-8-22(9-6-20)33(43)38-26-16-23-3-1-2-4-24(23)17-26/h1-6,8-11,18,21,26,29,37H,7,12-17,19H2,(H,38,43)(H,39,44)/t21?,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Biochemistry 45: 5964-73 (2006)
Article DOI: 10.1021/bi060173m
BindingDB Entry DOI: 10.7270/Q2W09450 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM14307
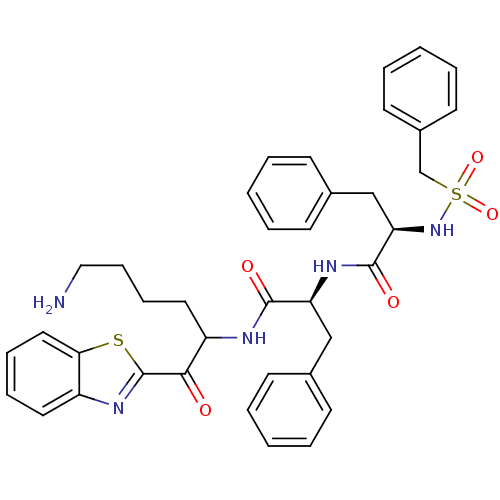
((2R)-N-[(1S)-1-{[6-amino-1-(1,3-benzothiazol-2-yl)...)Show SMILES NCCCCC(NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1ccccc1)NS(=O)(=O)Cc1ccccc1)C(=O)c1nc2ccccc2s1 |r| Show InChI InChI=1S/C38H41N5O5S2/c39-23-13-12-21-31(35(44)38-42-30-20-10-11-22-34(30)49-38)40-36(45)32(24-27-14-4-1-5-15-27)41-37(46)33(25-28-16-6-2-7-17-28)43-50(47,48)26-29-18-8-3-9-19-29/h1-11,14-20,22,31-33,43H,12-13,21,23-26,39H2,(H,40,45)(H,41,46)/t31?,32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | -43.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Biochemistry 45: 5964-73 (2006)
Article DOI: 10.1021/bi060173m
BindingDB Entry DOI: 10.7270/Q2W09450 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM14314
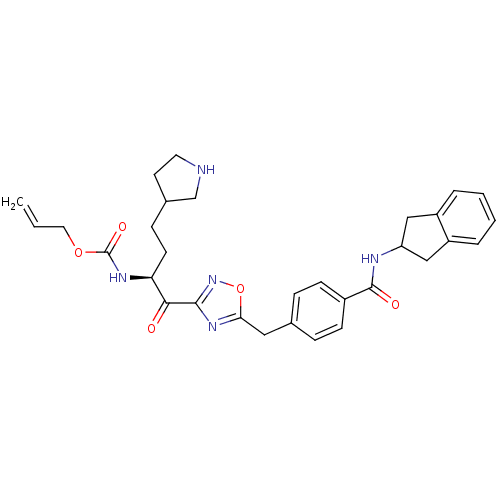
(CRA25 | prop-2-en-1-yl N-[(2S)-1-(5-{[4-(2,3-dihyd...)Show SMILES C=CCOC(=O)N[C@@H](CCC1CCNC1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NC2Cc3ccccc3C2)n1 |r| Show InChI InChI=1S/C31H35N5O5/c1-2-15-40-31(39)34-26(12-9-21-13-14-32-19-21)28(37)29-35-27(41-36-29)16-20-7-10-22(11-8-20)30(38)33-25-17-23-5-3-4-6-24(23)18-25/h2-8,10-11,21,25-26,32H,1,9,12-19H2,(H,33,38)(H,34,39)/t21?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Biochemistry 45: 5964-73 (2006)
Article DOI: 10.1021/bi060173m
BindingDB Entry DOI: 10.7270/Q2W09450 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187174
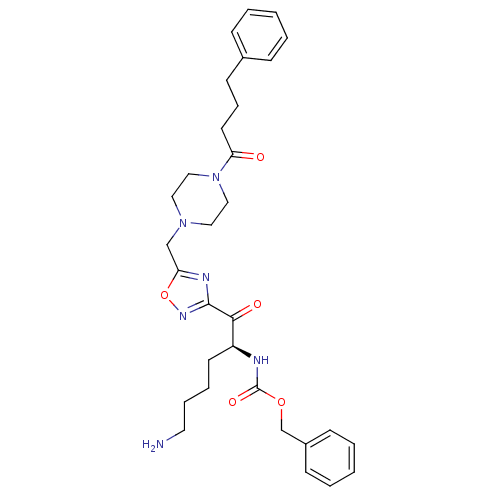
((S)-benzyl 6-amino-1-oxo-1-(5-((4-(4-phenylbutanoy...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(CN2CCN(CC2)C(=O)CCCc2ccccc2)n1 Show InChI InChI=1S/C31H40N6O5/c32-17-8-7-15-26(33-31(40)41-23-25-12-5-2-6-13-25)29(39)30-34-27(42-35-30)22-36-18-20-37(21-19-36)28(38)16-9-14-24-10-3-1-4-11-24/h1-6,10-13,26H,7-9,14-23,32H2,(H,33,40)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187156
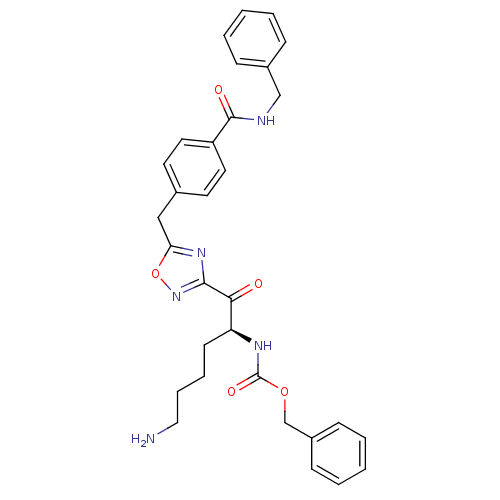
((S)-benzyl 1-(5-(4-(benzylcarbamoyl)benzyl)-1,2,4-...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCc2ccccc2)n1 Show InChI InChI=1S/C31H33N5O5/c32-18-8-7-13-26(34-31(39)40-21-24-11-5-2-6-12-24)28(37)29-35-27(41-36-29)19-22-14-16-25(17-15-22)30(38)33-20-23-9-3-1-4-10-23/h1-6,9-12,14-17,26H,7-8,13,18-21,32H2,(H,33,38)(H,34,39)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data