

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121552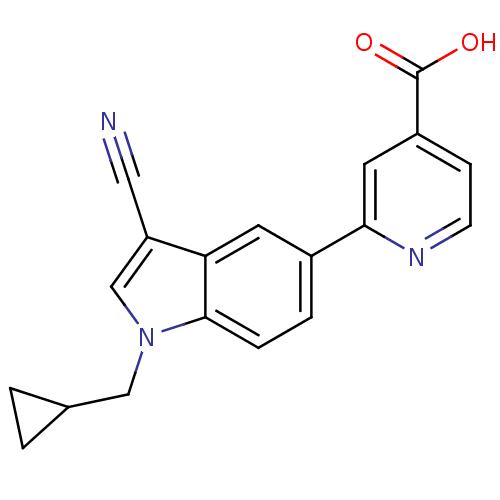 (US8729273, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121532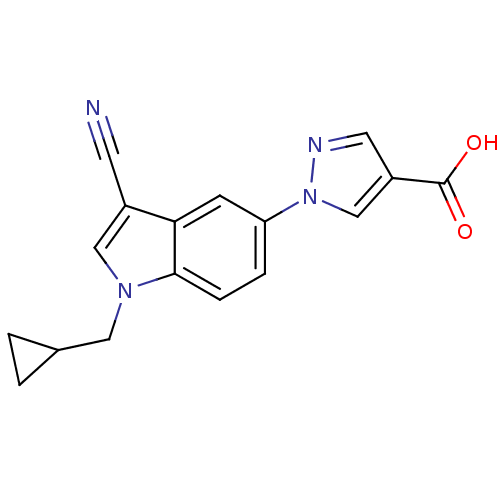 (US8729273, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121531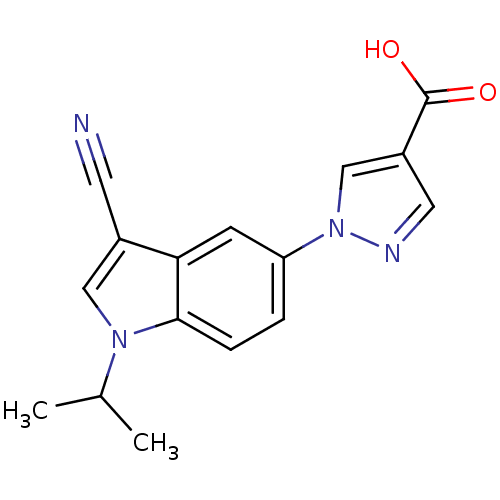 (US8729273, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121536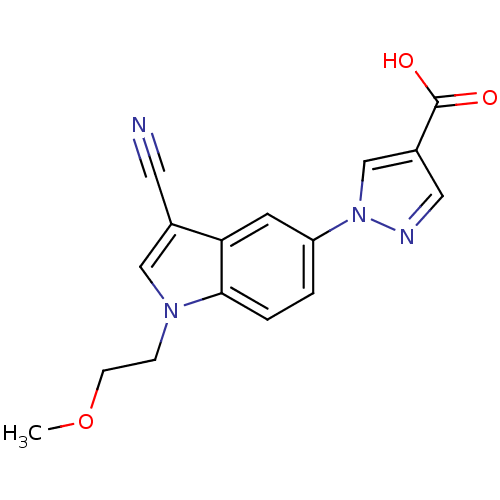 (US8729273, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121534 (US8729273, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121547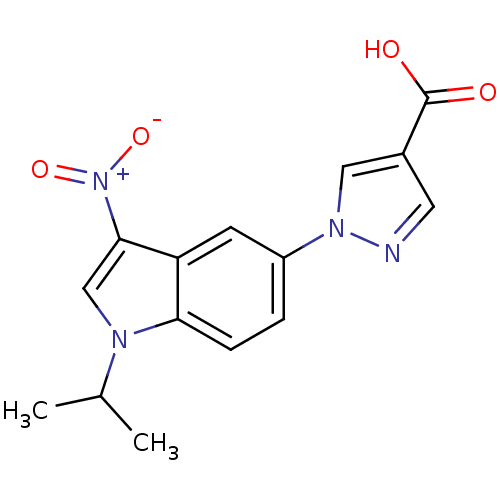 (US8729273, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121539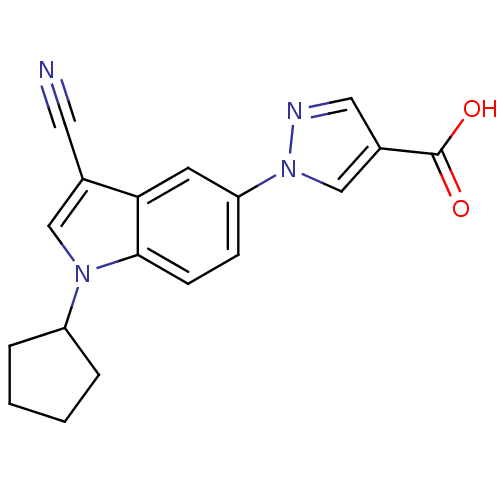 (US8729273, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121550 (US8729273, 20) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121543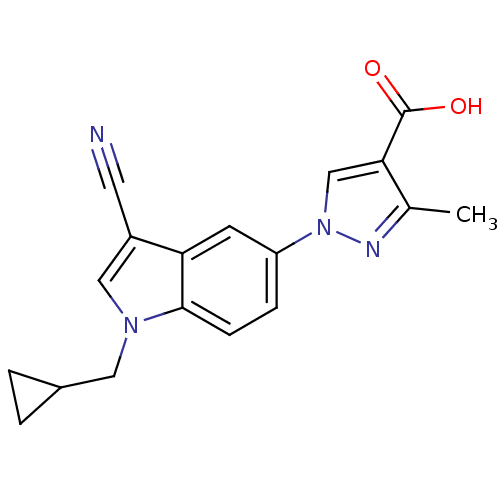 (US8729273, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121559 (US8729273, 29) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121535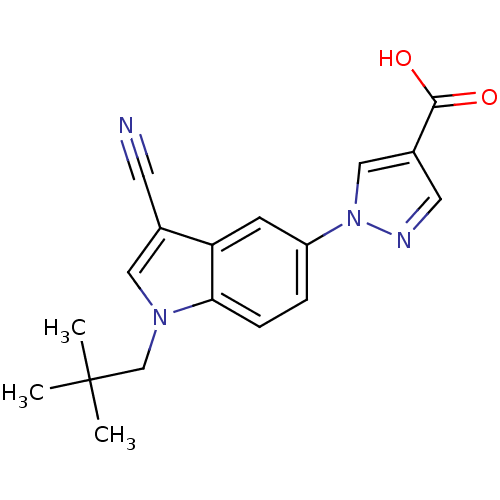 (US8729273, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121540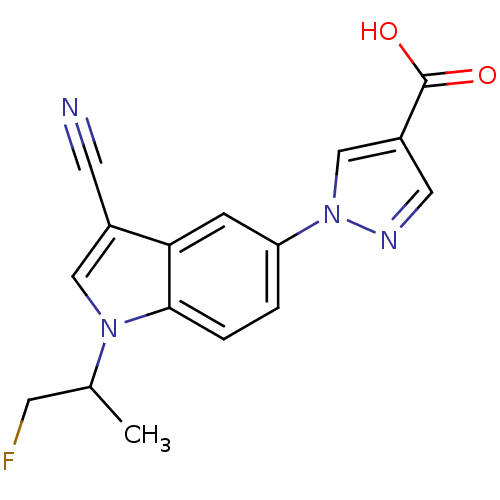 (US8729273, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121541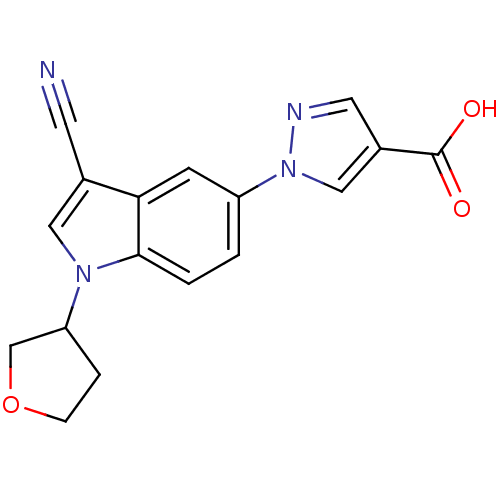 (US8729273, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121533 (US8729273, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121551 (US8729273, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121554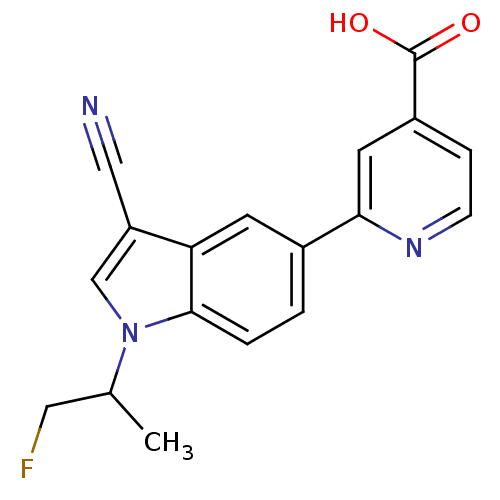 (US8729273, 24) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121537 (US8729273, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121538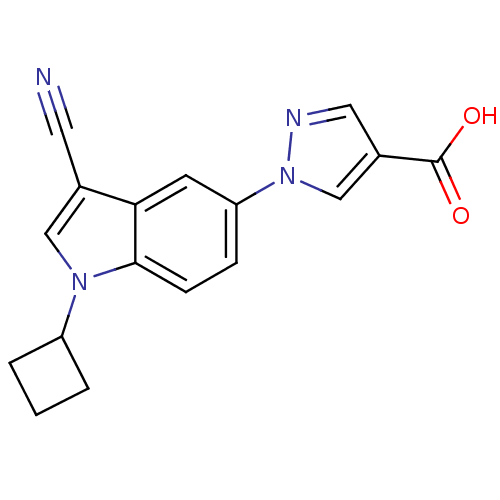 (US8729273, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121555 (US8729273, 25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214714 ((S)-2-(tert-butylamino)-1-(2-(2-(1-methylcyclohexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121553 (US8729273, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121557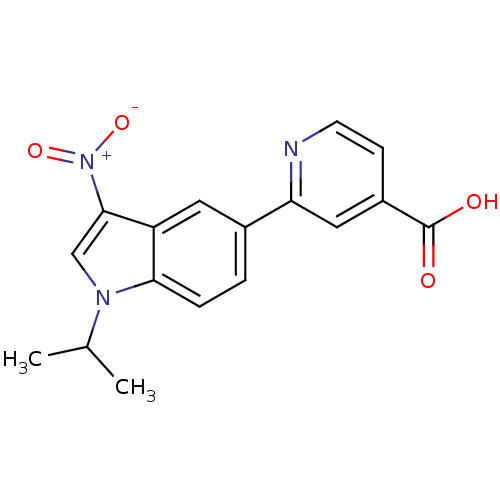 (US8729273, 27) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121542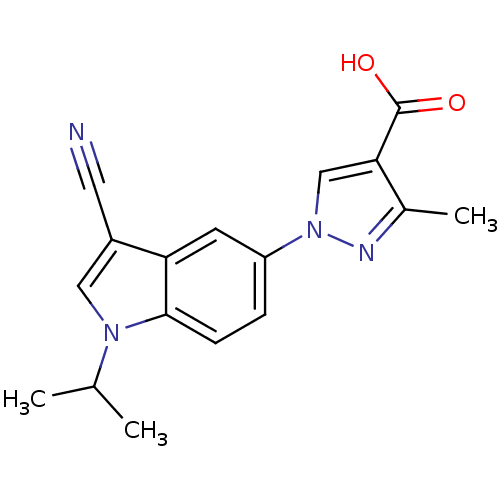 (US8729273, 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11.6 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121544 (US8729273, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121548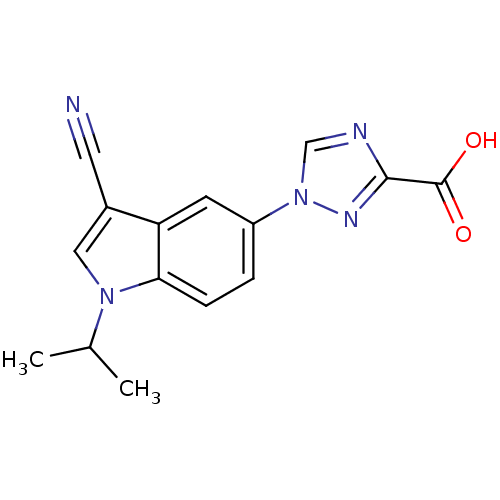 (US8729273, 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214713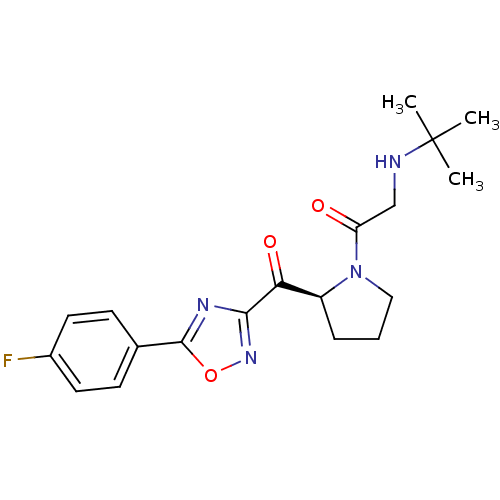 ((S)-2-(tert-butylamino)-1-(2-(5-(4-fluorophenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121560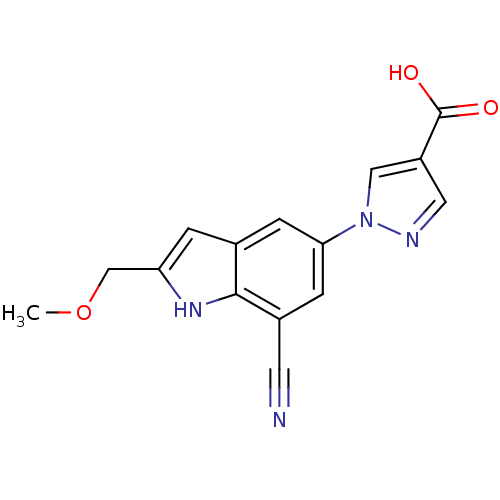 (US8729273, 30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214723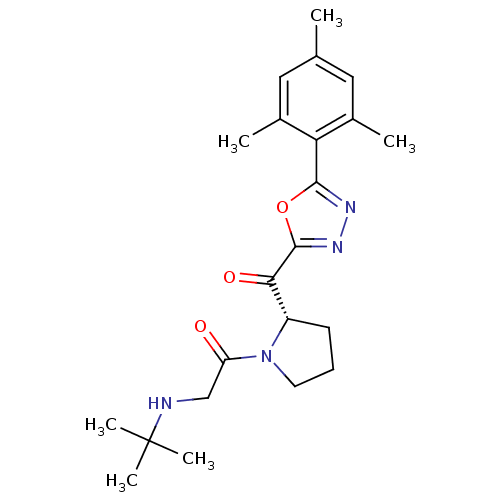 ((S)-2-(tert-butylamino)-1-(2-(2-mesityl-1,3,4-oxad...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214720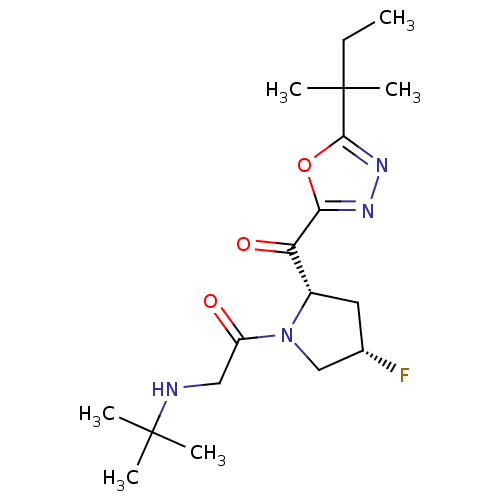 (2-(tert-butylamino)-1-((2S,4S)-4-fluoro-2-(2-tert-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214709 (1-((2S,4S)-2-(2-tert-butyl-1,3,4-oxadiazole-5-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121549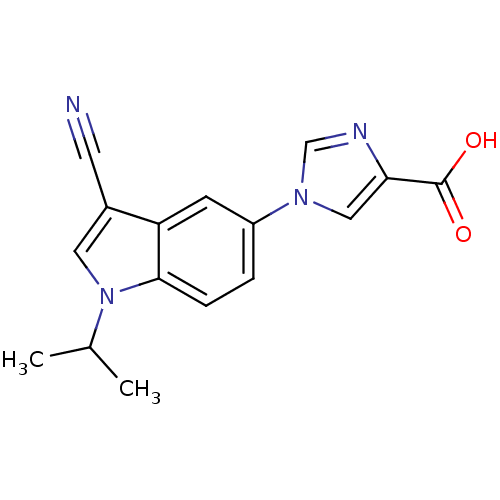 (US8729273, 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121558 (US8729273, 28) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214727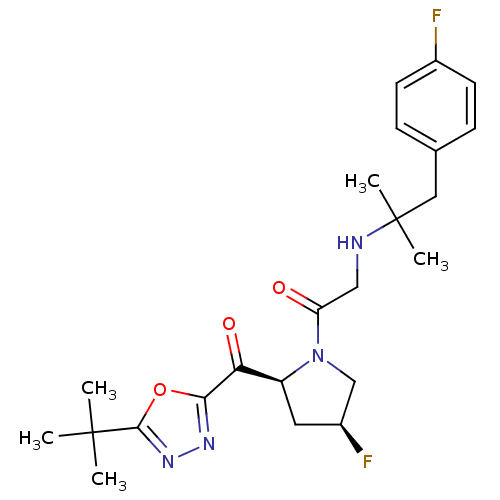 (1-((2S,4S)-2-(2-tert-butyl-1,3,4-oxadiazole-5-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214725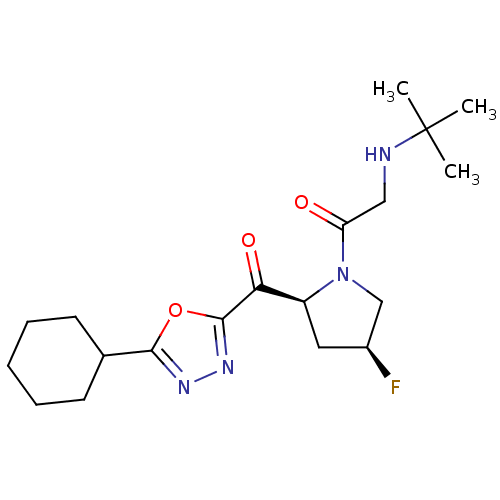 (2-(tert-butylamino)-1-((2S,4S)-2-(2-cyclohexyl-1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214726 ((S)-1-(2-(1,2,4-oxadiazole-3-carbonyl)pyrrolidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121561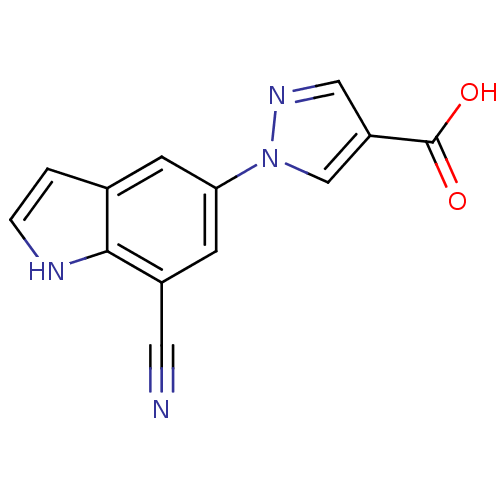 (US8729273, 31) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214712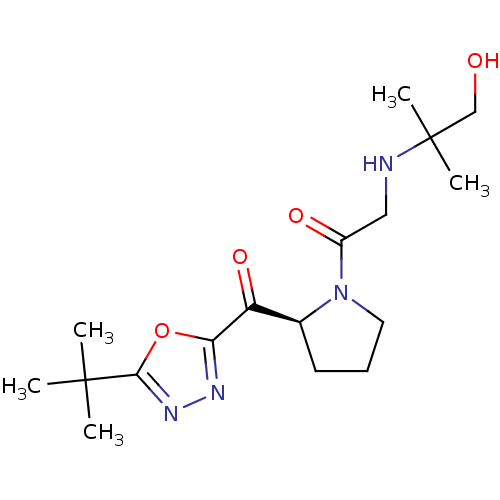 ((S)-1-(2-(2-tert-butyl-1,3,4-oxadiazole-5-carbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214719 (2-(adamantan-1-ylamino)-1-[(2S,4S)-4-fluoro-2-(5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214717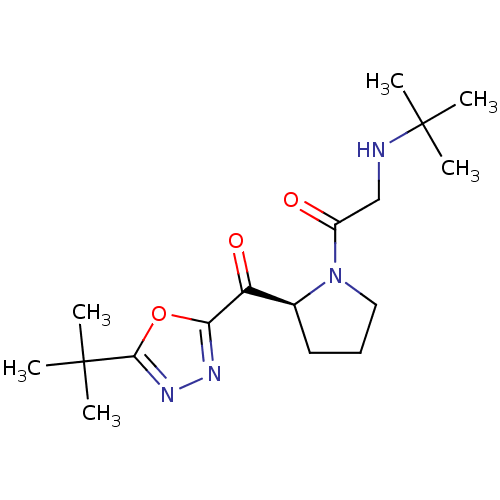 ((S)-1-(2-(2-tert-butyl-1,3,4-oxadiazole-5-carbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214718 (1-((2S,4S)-2-(5-tert-butyl-1,2,4-oxadiazole-3-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214724 (1-((2S,4S)-2-(5-tert-butyl-1,2,4-oxadiazole-3-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214722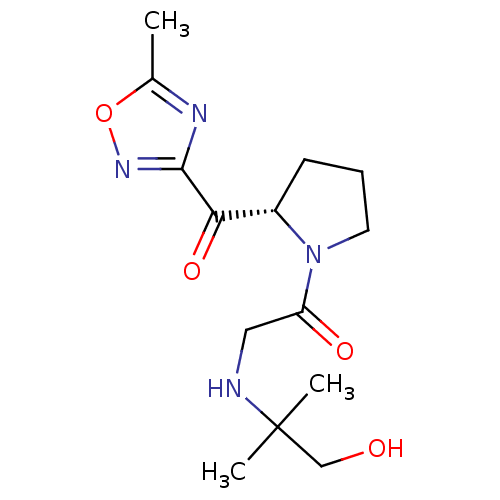 ((S)-2-(1-hydroxy-2-methylpropan-2-ylamino)-1-(2-(5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214728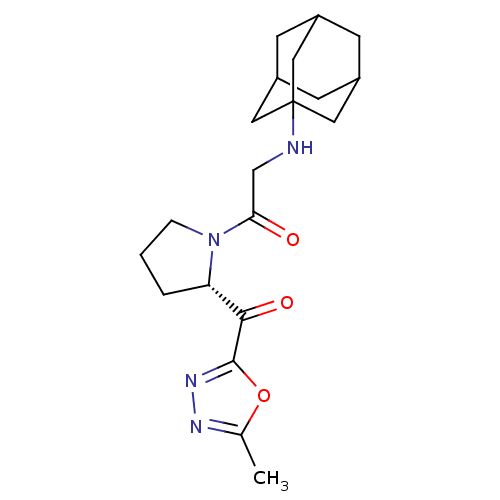 (2-(adamantan-1-ylamino)-1-[(S)-2-(5-methyl-[1,3,4]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214711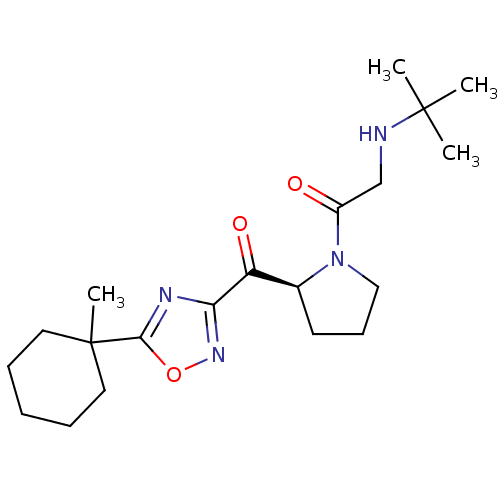 ((S)-2-(tert-butylamino)-1-(2-(5-(1-methylcyclohexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50214726 ((S)-1-(2-(1,2,4-oxadiazole-3-carbonyl)pyrrolidin-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of DPP2 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214729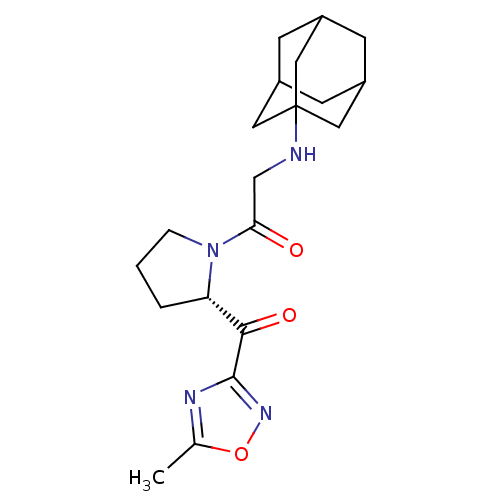 (2-(adamantan-1-ylamino)-1-[(S)-2-(5-methyl-[1,2,4]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214715 ((S)-1-(2-(5-tert-butyl-1,2,4-oxadiazole-3-carbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214710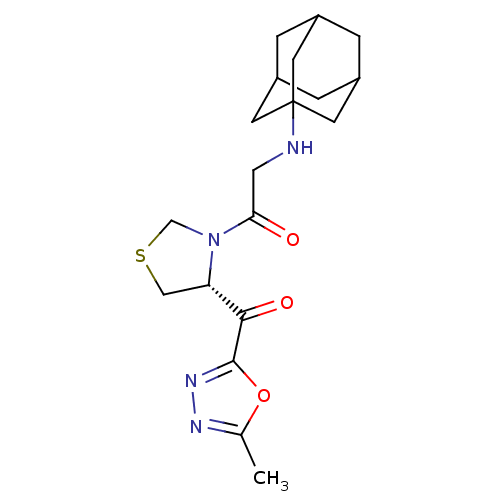 (2-(adamantan-1-ylamino)-1-[(R)-4-(5-methyl-[1,3,4]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214731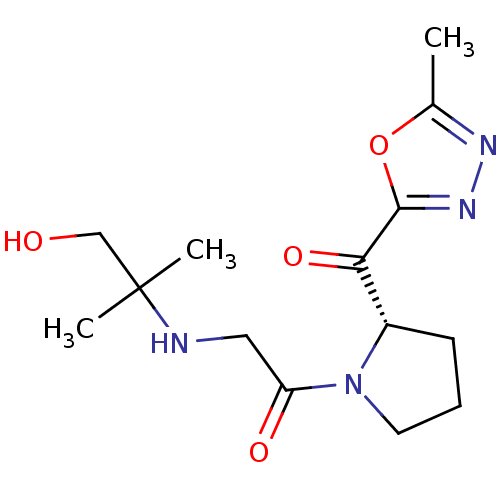 ((S)-2-(1-hydroxy-2-methylpropan-2-ylamino)-1-(2-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 255 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50214730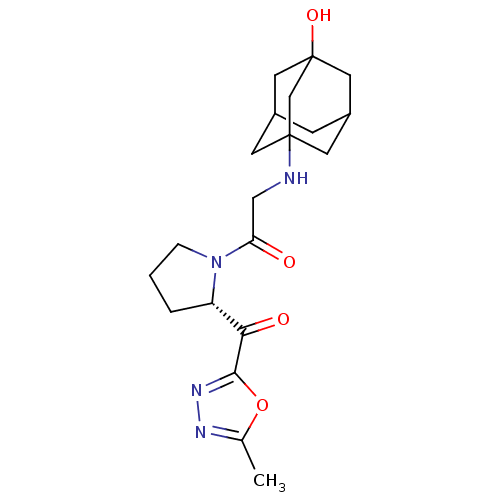 (2-(3-hydroxy-adamantan-1-ylamino)-1-[(S)-2-(5-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 339 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences, Ltd Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 4167-72 (2007) Article DOI: 10.1016/j.bmcl.2007.05.046 BindingDB Entry DOI: 10.7270/Q2611006 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 134 total ) | Next | Last >> |