| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2D6 |
|---|
| Ligand | BDBM50296762 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_582509 (CHEMBL1060842) |
|---|
| IC50 | >25000±n/a nM |
|---|
| Citation |  Nordhoff, S; López-Canet, M; Hoffmann-Enger, B; Bulat, S; Cerezo-Gálvez, S; Hill, O; Rosenbaum, C; Rummey, C; Thiemann, M; Matassa, VG; Edwards, PJ; Feurer, A From lead to preclinical candidate: optimization of beta-homophenylalanine based inhibitors of dipeptidyl peptidase IV. Bioorg Med Chem Lett19:4818-23 (2009) [PubMed] Article Nordhoff, S; López-Canet, M; Hoffmann-Enger, B; Bulat, S; Cerezo-Gálvez, S; Hill, O; Rosenbaum, C; Rummey, C; Thiemann, M; Matassa, VG; Edwards, PJ; Feurer, A From lead to preclinical candidate: optimization of beta-homophenylalanine based inhibitors of dipeptidyl peptidase IV. Bioorg Med Chem Lett19:4818-23 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2D6 |
|---|
| Name: | Cytochrome P450 2D6 |
|---|
| Synonyms: | CP2D6_HUMAN | CYP2D6 | CYP2DL1 | CYPIID6 | Cytochrome P450 2D6 (CYP2D6) | Debrisoquine 4-hydroxylase | P450-DB1 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 55774.82 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P10635 |
|---|
| Residue: | 497 |
|---|
| Sequence: | MGLEALVPLAVIVAIFLLLVDLMHRRQRWAARYPPGPLPLPGLGNLLHVDFQNTPYCFDQ
LRRRFGDVFSLQLAWTPVVVLNGLAAVREALVTHGEDTADRPPVPITQILGFGPRSQGVF
LARYGPAWREQRRFSVSTLRNLGLGKKSLEQWVTEEAACLCAAFANHSGRPFRPNGLLDK
AVSNVIASLTCGRRFEYDDPRFLRLLDLAQEGLKEESGFLREVLNAVPVLLHIPALAGKV
LRFQKAFLTQLDELLTEHRMTWDPAQPPRDLTEAFLAEMEKAKGNPESSFNDENLRIVVA
DLFSAGMVTTSTTLAWGLLLMILHPDVQRRVQQEIDDVIGQVRRPEMGDQAHMPYTTAVI
HEVQRFGDIVPLGVTHMTSRDIEVQGFRIPKGTTLITNLSSVLKDEAVWEKPFRFHPEHF
LDAQGHFVKPEAFLPFSAGRRACLGEPLARMELFLFFTSLLQHFSFSVPTGQPRPSHHGV
FAFLVSPSPYELCAVPR
|
|
|
|---|
| BDBM50296762 |
|---|
| n/a |
|---|
| Name | BDBM50296762 |
|---|
| Synonyms: | CHEMBL550114 | N-(((S)-1-((R)-3-amino-4-(2,4,5-trifluorophenyl)butanoyl)pyrrolidin-2-yl)methyl)-3,3,3-trifluoropropanamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H21F6N3O2 |
|---|
| Mol. Mass. | 425.3687 |
|---|
| SMILES | N[C@@H](CC(=O)N1CCC[C@H]1CNC(=O)CC(F)(F)F)Cc1cc(F)c(F)cc1F |r| |
|---|
| Structure | 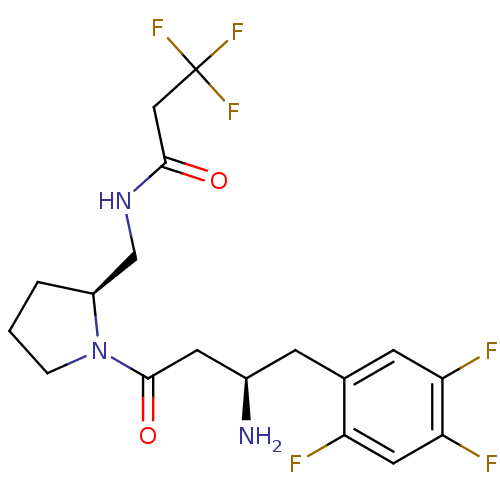
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Nordhoff, S; López-Canet, M; Hoffmann-Enger, B; Bulat, S; Cerezo-Gálvez, S; Hill, O; Rosenbaum, C; Rummey, C; Thiemann, M; Matassa, VG; Edwards, PJ; Feurer, A From lead to preclinical candidate: optimization of beta-homophenylalanine based inhibitors of dipeptidyl peptidase IV. Bioorg Med Chem Lett19:4818-23 (2009) [PubMed] Article
Nordhoff, S; López-Canet, M; Hoffmann-Enger, B; Bulat, S; Cerezo-Gálvez, S; Hill, O; Rosenbaum, C; Rummey, C; Thiemann, M; Matassa, VG; Edwards, PJ; Feurer, A From lead to preclinical candidate: optimization of beta-homophenylalanine based inhibitors of dipeptidyl peptidase IV. Bioorg Med Chem Lett19:4818-23 (2009) [PubMed] Article