| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50336079 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_718098 (CHEMBL1671551) |
|---|
| IC50 | 2751±n/a nM |
|---|
| Citation |  Huang, C; Moree, WJ; Zamani-Kord, S; Li, BF; Tucci, FC; Malany, S; Wen, J; Wang, H; Hoare, SR; Yang, C; Madan, A; Crowe, PD; Beaton, G Influence of pKa on the biotransformation of indene H1-antihistamines by CYP2D6. Bioorg Med Chem Lett21:947-51 (2011) [PubMed] Article Huang, C; Moree, WJ; Zamani-Kord, S; Li, BF; Tucci, FC; Malany, S; Wen, J; Wang, H; Hoare, SR; Yang, C; Madan, A; Crowe, PD; Beaton, G Influence of pKa on the biotransformation of indene H1-antihistamines by CYP2D6. Bioorg Med Chem Lett21:947-51 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50336079 |
|---|
| n/a |
|---|
| Name | BDBM50336079 |
|---|
| Synonyms: | (R)-N-(furan-2-ylmethyl)-N-methyl-2-(3-(1-(pyrazin-2-yl)ethyl)-1H-inden-2-yl)ethanamine | CHEMBL1669418 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H25N3O |
|---|
| Mol. Mass. | 359.4641 |
|---|
| SMILES | C[C@H](C1=C(CCN(C)Cc2ccco2)Cc2ccccc12)c1cnccn1 |r,c:2| |
|---|
| Structure | 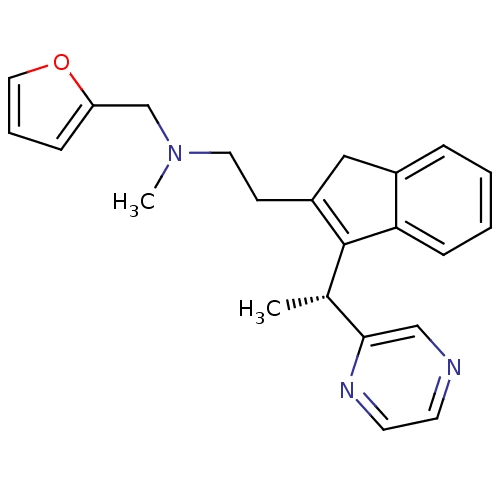
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Huang, C; Moree, WJ; Zamani-Kord, S; Li, BF; Tucci, FC; Malany, S; Wen, J; Wang, H; Hoare, SR; Yang, C; Madan, A; Crowe, PD; Beaton, G Influence of pKa on the biotransformation of indene H1-antihistamines by CYP2D6. Bioorg Med Chem Lett21:947-51 (2011) [PubMed] Article
Huang, C; Moree, WJ; Zamani-Kord, S; Li, BF; Tucci, FC; Malany, S; Wen, J; Wang, H; Hoare, SR; Yang, C; Madan, A; Crowe, PD; Beaton, G Influence of pKa on the biotransformation of indene H1-antihistamines by CYP2D6. Bioorg Med Chem Lett21:947-51 (2011) [PubMed] Article