 Found 448 hits with Last Name = 'zamani-kord' and Initial = 's'
Found 448 hits with Last Name = 'zamani-kord' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50297306
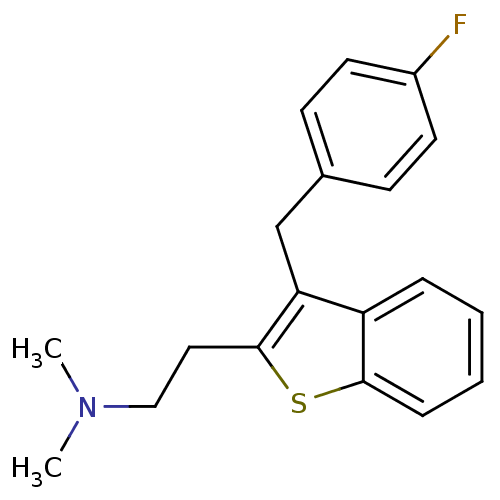
(CHEMBL540982 | {2-[3-(4-Fluoro-benzyl)-benzo[b]thi...)Show InChI InChI=1S/C19H20FNS/c1-21(2)12-11-19-17(13-14-7-9-15(20)10-8-14)16-5-3-4-6-18(16)22-19/h3-10H,11-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in HEK293 Flp-In cells by liquid scintillation counting |
J Med Chem 52: 5307-10 (2009)
Article DOI: 10.1021/jm900933k
BindingDB Entry DOI: 10.7270/Q2057G0S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50297304
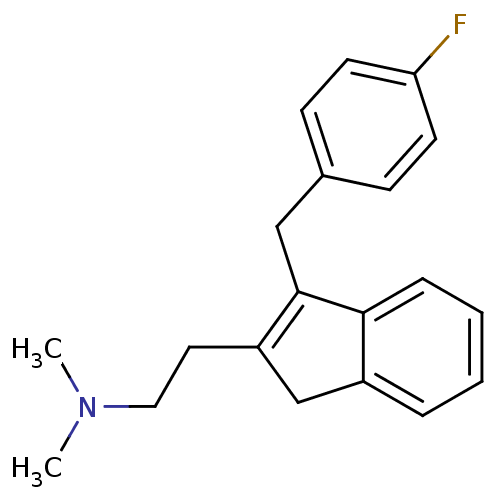
(CHEMBL560741 | {2-[3-(4-Fluoro-benzyl)-1H-inden-2-...)Show InChI InChI=1S/C20H22FN/c1-22(2)12-11-17-14-16-5-3-4-6-19(16)20(17)13-15-7-9-18(21)10-8-15/h3-10H,11-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in HEK293 Flp-In cells by liquid scintillation counting |
J Med Chem 52: 5307-10 (2009)
Article DOI: 10.1021/jm900933k
BindingDB Entry DOI: 10.7270/Q2057G0S |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315206
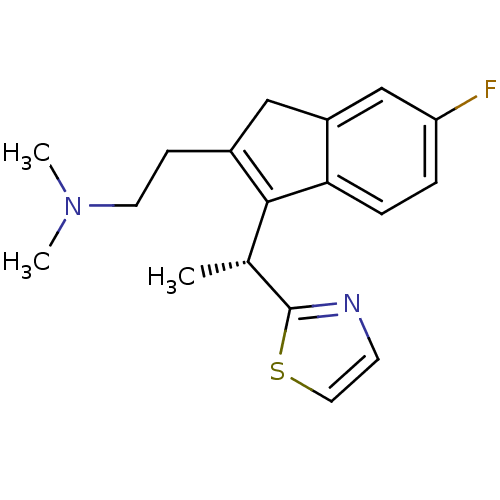
((R)-2-(6-fluoro-3-(1-(thiazol-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)C)Cc2cc(F)ccc12)c1nccs1 |r,c:2| Show InChI InChI=1S/C18H21FN2S/c1-12(18-20-7-9-22-18)17-13(6-8-21(2)3)10-14-11-15(19)4-5-16(14)17/h4-5,7,9,11-12H,6,8,10H2,1-3H3/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50297310
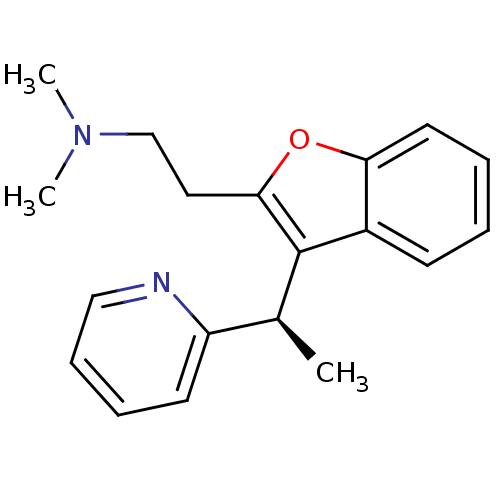
((-)-Dimethyl-{2-[3-((R)-1-pyridin-2-yl-ethyl)-benz...)Show InChI InChI=1S/C19H22N2O/c1-14(16-9-6-7-12-20-16)19-15-8-4-5-10-17(15)22-18(19)11-13-21(2)3/h4-10,12,14H,11,13H2,1-3H3/t14-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from human muscarinic M1 receptor expressed in CHO Flp-In cells by liquid scintillation counting |
J Med Chem 52: 5307-10 (2009)
Article DOI: 10.1021/jm900933k
BindingDB Entry DOI: 10.7270/Q2057G0S |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297307
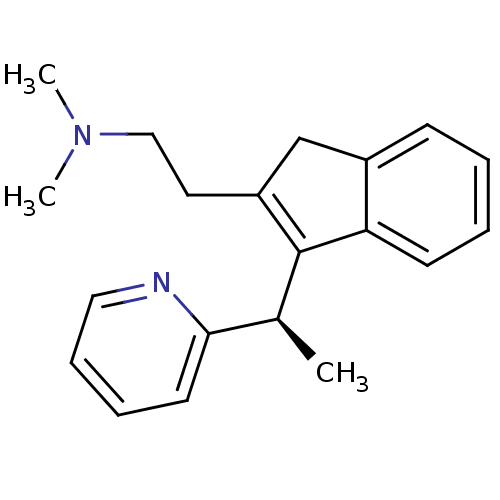
(CHEMBL564226 | R-dimethindene)Show SMILES C[C@H](C1=C(CCN(C)C)Cc2ccccc12)c1ccccn1 |r,c:2| Show InChI InChI=1S/C20H24N2/c1-15(19-10-6-7-12-21-19)20-17(11-13-22(2)3)14-16-8-4-5-9-18(16)20/h4-10,12,15H,11,13-14H2,1-3H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation counting |
J Med Chem 52: 5307-10 (2009)
Article DOI: 10.1021/jm900933k
BindingDB Entry DOI: 10.7270/Q2057G0S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50297305
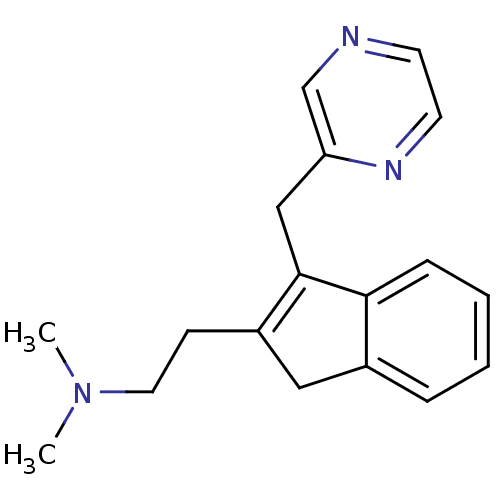
(CHEMBL559251 | Dimethyl-[2-(3-pyrazin-2ylmethyl-1H...)Show InChI InChI=1S/C18H21N3/c1-21(2)10-7-15-11-14-5-3-4-6-17(14)18(15)12-16-13-19-8-9-20-16/h3-6,8-9,13H,7,10-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in HEK293 Flp-In cells by liquid scintillation counting |
J Med Chem 52: 5307-10 (2009)
Article DOI: 10.1021/jm900933k
BindingDB Entry DOI: 10.7270/Q2057G0S |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297304

(CHEMBL560741 | {2-[3-(4-Fluoro-benzyl)-1H-inden-2-...)Show InChI InChI=1S/C20H22FN/c1-22(2)12-11-17-14-16-5-3-4-6-19(16)20(17)13-15-7-9-18(21)10-8-15/h3-10H,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation counting |
J Med Chem 52: 5307-10 (2009)
Article DOI: 10.1021/jm900933k
BindingDB Entry DOI: 10.7270/Q2057G0S |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315205
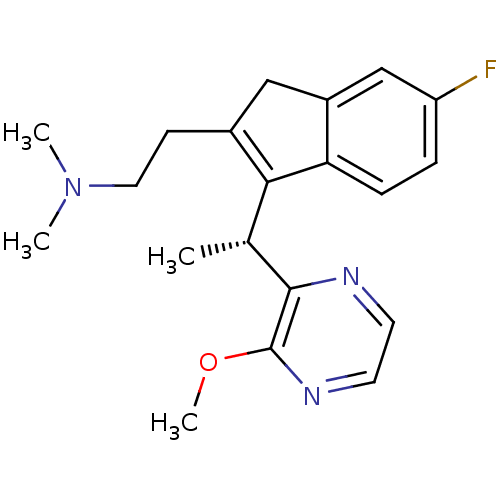
((R)-2-(6-fluoro-3-(1-(3-methoxypyrazin-2-yl)ethyl)...)Show SMILES COc1nccnc1[C@H](C)C1=C(CCN(C)C)Cc2cc(F)ccc12 |r,c:11| Show InChI InChI=1S/C20H24FN3O/c1-13(19-20(25-4)23-9-8-22-19)18-14(7-10-24(2)3)11-15-12-16(21)5-6-17(15)18/h5-6,8-9,12-13H,7,10-11H2,1-4H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of histamine H1 receptor |
Bioorg Med Chem Lett 20: 5874-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.117
BindingDB Entry DOI: 10.7270/Q26W9BDC |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315205

((R)-2-(6-fluoro-3-(1-(3-methoxypyrazin-2-yl)ethyl)...)Show SMILES COc1nccnc1[C@H](C)C1=C(CCN(C)C)Cc2cc(F)ccc12 |r,c:11| Show InChI InChI=1S/C20H24FN3O/c1-13(19-20(25-4)23-9-8-22-19)18-14(7-10-24(2)3)11-15-12-16(21)5-6-17(15)18/h5-6,8-9,12-13H,7,10-11H2,1-4H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315198
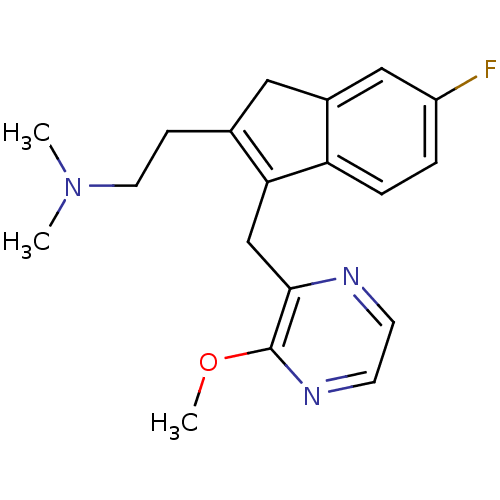
(2-(6-fluoro-3-((3-methoxypyrazin-2-yl)methyl)-1H-i...)Show SMILES COc1nccnc1CC1=C(CCN(C)C)Cc2cc(F)ccc12 |c:10| Show InChI InChI=1S/C19H22FN3O/c1-23(2)9-6-13-10-14-11-15(20)4-5-16(14)17(13)12-18-19(24-3)22-8-7-21-18/h4-5,7-8,11H,6,9-10,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315191
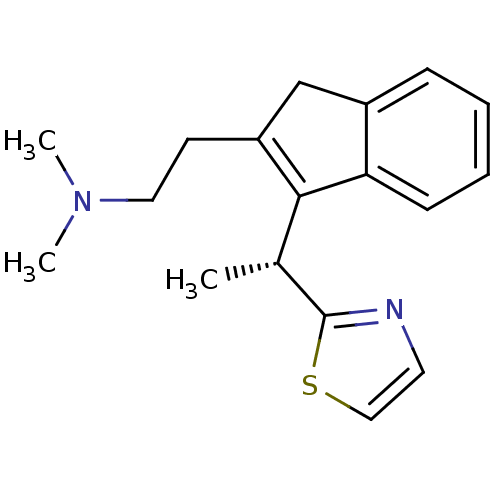
((R)-N,N-dimethyl-2-(3-(1-(thiazol-2-yl)ethyl)-1H-i...)Show SMILES C[C@H](C1=C(CCN(C)C)Cc2ccccc12)c1nccs1 |r,c:2| Show InChI InChI=1S/C18H22N2S/c1-13(18-19-9-11-21-18)17-15(8-10-20(2)3)12-14-6-4-5-7-16(14)17/h4-7,9,11,13H,8,10,12H2,1-3H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297310

((-)-Dimethyl-{2-[3-((R)-1-pyridin-2-yl-ethyl)-benz...)Show InChI InChI=1S/C19H22N2O/c1-14(16-9-6-7-12-20-16)19-15-8-4-5-10-17(15)22-18(19)11-13-21(2)3/h4-10,12,14H,11,13H2,1-3H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation counting |
J Med Chem 52: 5307-10 (2009)
Article DOI: 10.1021/jm900933k
BindingDB Entry DOI: 10.7270/Q2057G0S |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315190

((R)-2-(3-(1-(3-methoxypyrazin-2-yl)ethyl)-1H-inden...)Show SMILES COc1nccnc1[C@H](C)C1=C(CCN(C)C)Cc2ccccc12 |r,c:11| Show InChI InChI=1S/C20H25N3O/c1-14(19-20(24-4)22-11-10-21-19)18-16(9-12-23(2)3)13-15-7-5-6-8-17(15)18/h5-8,10-11,14H,9,12-13H2,1-4H3/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50171332

(1-(2-Bromo-4-isopropyl-phenyl)-7-methyl-5-(1-propy...)Show SMILES CCCC(CCC)N1CCn2c3c1cc(C)nc3n(-c1ccc(cc1Br)C(C)C)c2=O |(-5.8,2.54,;-4.46,3.31,;-3.13,2.54,;-1.79,3.31,;-1.78,4.86,;-.44,5.63,;-.44,7.18,;-.44,2.52,;.9,3.29,;2.23,2.52,;2.22,.97,;.89,.2,;-.44,.97,;-1.78,.19,;-1.78,-1.36,;-3.27,-1.8,;.08,-2.35,;1.22,-1.32,;2.76,-1.46,;3.73,-2.67,;3.16,-4.1,;4.14,-5.31,;5.66,-5.06,;6.2,-3.63,;5.25,-2.44,;5.81,-1.01,;6.41,-6.39,;5.64,-7.72,;7.96,-6.41,;3.37,-.05,;4.89,.27,)| Show InChI InChI=1S/C25H33BrN4O/c1-6-8-19(9-7-2)28-12-13-29-23-22(28)14-17(5)27-24(23)30(25(29)31)21-11-10-18(16(3)4)15-20(21)26/h10-11,14-16,19H,6-9,12-13H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for corticotropin-releasing factor-1 expressed in leukocyte tyrosine kinase cells |
J Med Chem 48: 5104-7 (2005)
Article DOI: 10.1021/jm050384+
BindingDB Entry DOI: 10.7270/Q2610ZVM |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315204

((R)-2-(6-methoxy-3-(1-(3-methoxypyrazin-2-yl)ethyl...)Show SMILES COc1ccc2C([C@@H](C)c3nccnc3OC)=C(CCN(C)C)Cc2c1 |r,t:17| Show InChI InChI=1S/C21H27N3O2/c1-14(20-21(26-5)23-10-9-22-20)19-15(8-11-24(2)3)12-16-13-17(25-4)6-7-18(16)19/h6-7,9-10,13-14H,8,11-12H2,1-5H3/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297308
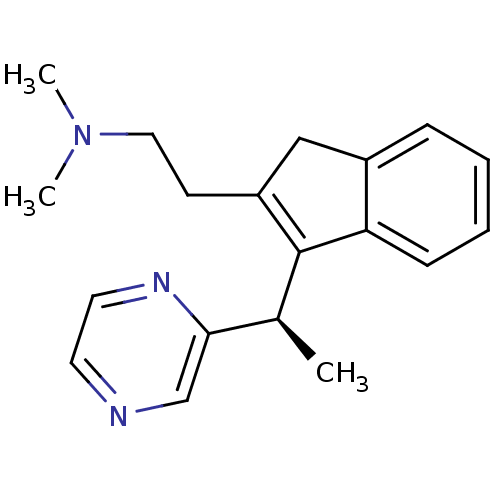
((-)-Dimethyl-{2-[3-((R)-1-pyrazin-2-yl-ethyl)-1H-i...)Show SMILES C[C@H](C1=C(CCN(C)C)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C19H23N3/c1-14(18-13-20-9-10-21-18)19-16(8-11-22(2)3)12-15-6-4-5-7-17(15)19/h4-7,9-10,13-14H,8,11-12H2,1-3H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297308

((-)-Dimethyl-{2-[3-((R)-1-pyrazin-2-yl-ethyl)-1H-i...)Show SMILES C[C@H](C1=C(CCN(C)C)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C19H23N3/c1-14(18-13-20-9-10-21-18)19-16(8-11-22(2)3)12-15-6-4-5-7-17(15)19/h4-7,9-10,13-14H,8,11-12H2,1-3H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation counting |
J Med Chem 52: 5307-10 (2009)
Article DOI: 10.1021/jm900933k
BindingDB Entry DOI: 10.7270/Q2057G0S |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297852
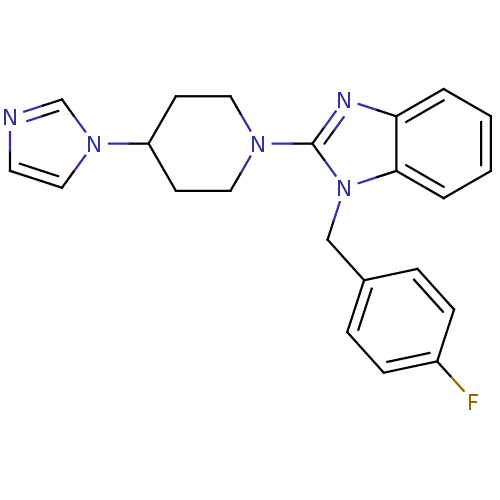
(2-(4-(1H-imidazol-1-yl)piperidin-1-yl)-1-(4-fluoro...)Show SMILES Fc1ccc(Cn2c(nc3ccccc23)N2CCC(CC2)n2ccnc2)cc1 Show InChI InChI=1S/C22H22FN5/c23-18-7-5-17(6-8-18)15-28-21-4-2-1-3-20(21)25-22(28)26-12-9-19(10-13-26)27-14-11-24-16-27/h1-8,11,14,16,19H,9-10,12-13,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297308

((-)-Dimethyl-{2-[3-((R)-1-pyrazin-2-yl-ethyl)-1H-i...)Show SMILES C[C@H](C1=C(CCN(C)C)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C19H23N3/c1-14(18-13-20-9-10-21-18)19-16(8-11-22(2)3)12-15-6-4-5-7-17(15)19/h4-7,9-10,13-14H,8,11-12H2,1-3H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315195
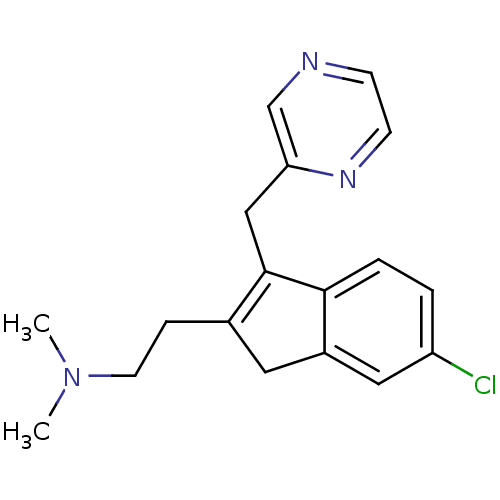
(2-(6-chloro-3-(pyrazin-2-ylmethyl)-1H-inden-2-yl)-...)Show InChI InChI=1S/C18H20ClN3/c1-22(2)8-5-13-9-14-10-15(19)3-4-17(14)18(13)11-16-12-20-6-7-21-16/h3-4,6-7,10,12H,5,8-9,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315196
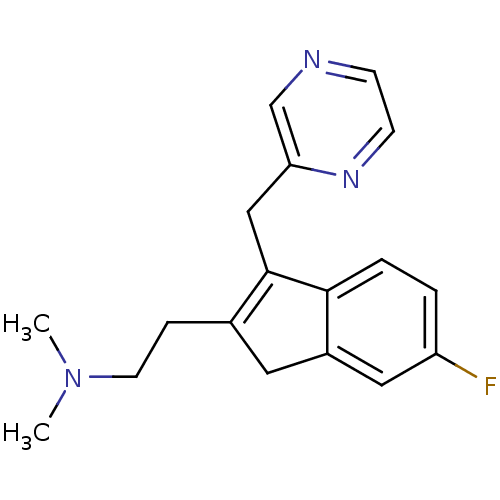
(2-(6-fluoro-3-(pyrazin-2-ylmethyl)-1H-inden-2-yl)-...)Show InChI InChI=1S/C18H20FN3/c1-22(2)8-5-13-9-14-10-15(19)3-4-17(14)18(13)11-16-12-20-6-7-21-16/h3-4,6-7,10,12H,5,8-9,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297308

((-)-Dimethyl-{2-[3-((R)-1-pyrazin-2-yl-ethyl)-1H-i...)Show SMILES C[C@H](C1=C(CCN(C)C)Cc2ccccc12)c1cnccn1 |r,c:2| Show InChI InChI=1S/C19H23N3/c1-14(18-13-20-9-10-21-18)19-16(8-11-22(2)3)12-15-6-4-5-7-17(15)19/h4-7,9-10,13-14H,8,11-12H2,1-3H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of histamine H1 receptor |
Bioorg Med Chem Lett 20: 5874-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.117
BindingDB Entry DOI: 10.7270/Q26W9BDC |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315197

(2-(6-chloro-3-((3-methoxypyrazin-2-yl)methyl)-1H-i...)Show SMILES COc1nccnc1CC1=C(CCN(C)C)Cc2cc(Cl)ccc12 |c:10| Show InChI InChI=1S/C19H22ClN3O/c1-23(2)9-6-13-10-14-11-15(20)4-5-16(14)17(13)12-18-19(24-3)22-8-7-21-18/h4-5,7-8,11H,6,9-10,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297863
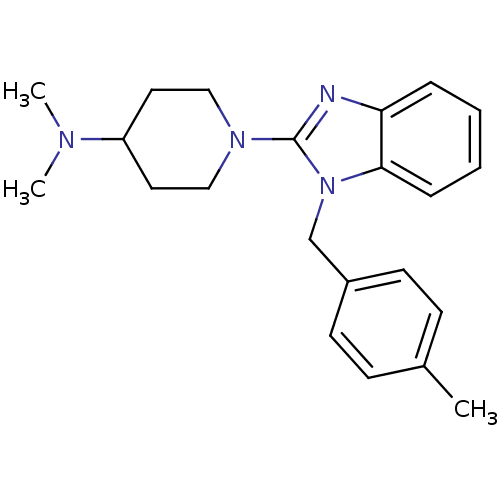
(CHEMBL563451 | N,N-dimethyl-1-(1-(4-methylbenzyl)-...)Show InChI InChI=1S/C22H28N4/c1-17-8-10-18(11-9-17)16-26-21-7-5-4-6-20(21)23-22(26)25-14-12-19(13-15-25)24(2)3/h4-11,19H,12-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315200

(2-(6-fluoro-3-(pyridazin-3-ylmethyl)-1H-inden-2-yl...)Show InChI InChI=1S/C18H20FN3/c1-22(2)9-7-13-10-14-11-15(19)5-6-17(14)18(13)12-16-4-3-8-20-21-16/h3-6,8,11H,7,9-10,12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315200

(2-(6-fluoro-3-(pyridazin-3-ylmethyl)-1H-inden-2-yl...)Show InChI InChI=1S/C18H20FN3/c1-22(2)9-7-13-10-14-11-15(19)5-6-17(14)18(13)12-16-4-3-8-20-21-16/h3-6,8,11H,7,9-10,12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of histamine H1 receptor |
Bioorg Med Chem Lett 20: 5874-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.117
BindingDB Entry DOI: 10.7270/Q26W9BDC |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297864
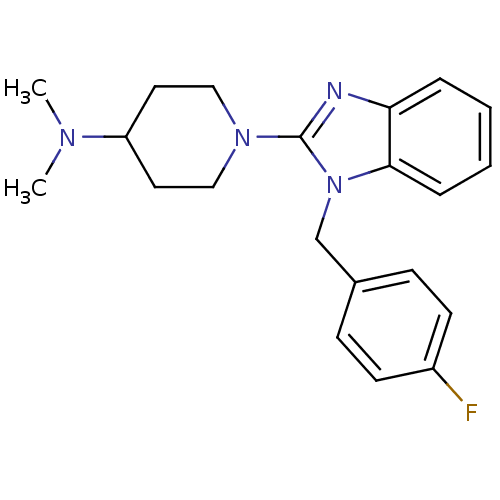
(1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N,...)Show InChI InChI=1S/C21H25FN4/c1-24(2)18-11-13-25(14-12-18)21-23-19-5-3-4-6-20(19)26(21)15-16-7-9-17(22)10-8-16/h3-10,18H,11-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50171318

(1-(4-Methoxy-phenyl)-7-methyl-5-(1-propyl-butyl)-4...)Show SMILES CCCC(CCC)N1CCn2c3c1cc(C)nc3n(-c1ccc(OC)cc1)c2=O Show InChI InChI=1S/C23H30N4O2/c1-5-7-17(8-6-2)25-13-14-26-21-20(25)15-16(3)24-22(21)27(23(26)28)18-9-11-19(29-4)12-10-18/h9-12,15,17H,5-8,13-14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for corticotropin-releasing factor-1 expressed in leukocyte tyrosine kinase cells |
J Med Chem 48: 5104-7 (2005)
Article DOI: 10.1021/jm050384+
BindingDB Entry DOI: 10.7270/Q2610ZVM |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50297306

(CHEMBL540982 | {2-[3-(4-Fluoro-benzyl)-benzo[b]thi...)Show InChI InChI=1S/C19H20FNS/c1-21(2)12-11-19-17(13-14-7-9-15(20)10-8-14)16-5-3-4-6-18(16)22-19/h3-10H,11-13H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from human muscarinic M1 receptor expressed in CHO Flp-In cells by liquid scintillation counting |
J Med Chem 52: 5307-10 (2009)
Article DOI: 10.1021/jm900933k
BindingDB Entry DOI: 10.7270/Q2057G0S |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315199
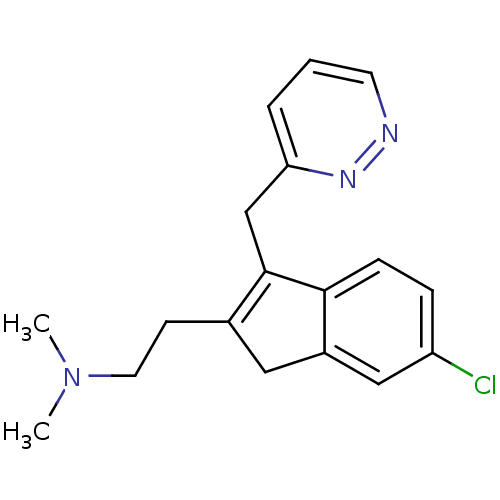
(2-(6-chloro-3-(pyridazin-3-ylmethyl)-1H-inden-2-yl...)Show InChI InChI=1S/C18H20ClN3/c1-22(2)9-7-13-10-14-11-15(19)5-6-17(14)18(13)12-16-4-3-8-20-21-16/h3-6,8,11H,7,9-10,12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297859
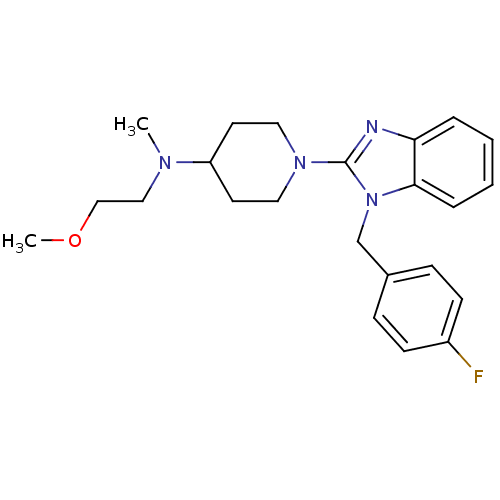
(1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...)Show SMILES COCCN(C)C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1 Show InChI InChI=1S/C23H29FN4O/c1-26(15-16-29-2)20-11-13-27(14-12-20)23-25-21-5-3-4-6-22(21)28(23)17-18-7-9-19(24)10-8-18/h3-10,20H,11-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315187
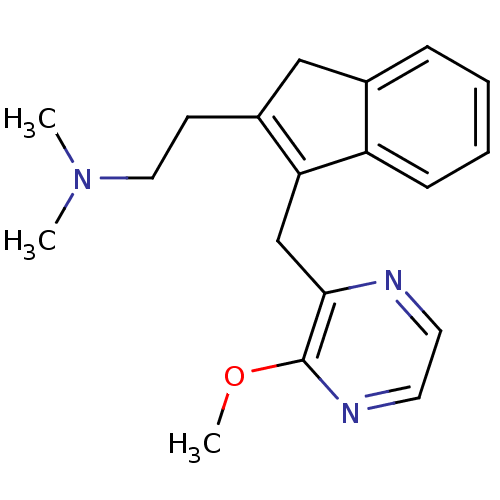
(2-(3-((3-methoxypyrazin-2-yl)methyl)-1H-inden-2-yl...)Show InChI InChI=1S/C19H23N3O/c1-22(2)11-8-15-12-14-6-4-5-7-16(14)17(15)13-18-19(23-3)21-10-9-20-18/h4-7,9-10H,8,11-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315207

((R)-N,N-dimethyl-2-(6-methyl-3-(1-(thiazol-2-yl)et...)Show SMILES C[C@H](C1=C(CCN(C)C)Cc2cc(C)ccc12)c1nccs1 |r,c:2| Show InChI InChI=1S/C19H24N2S/c1-13-5-6-17-16(11-13)12-15(7-9-21(3)4)18(17)14(2)19-20-8-10-22-19/h5-6,8,10-11,14H,7,9,12H2,1-4H3/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297862
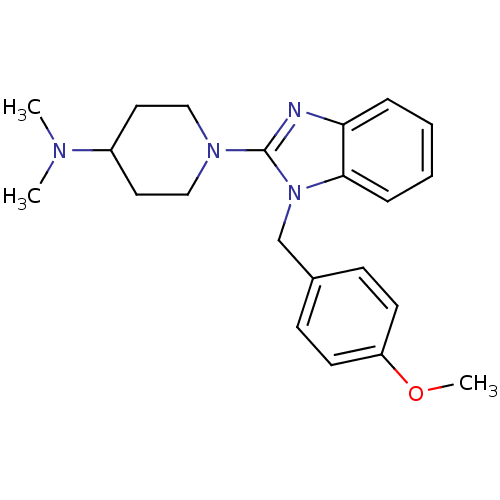
(1-(1-(4-methoxybenzyl)-1H-benzo[d]imidazol-2-yl)-N...)Show SMILES COc1ccc(Cn2c(nc3ccccc23)N2CCC(CC2)N(C)C)cc1 Show InChI InChI=1S/C22H28N4O/c1-24(2)18-12-14-25(15-13-18)22-23-20-6-4-5-7-21(20)26(22)16-17-8-10-19(27-3)11-9-17/h4-11,18H,12-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297869
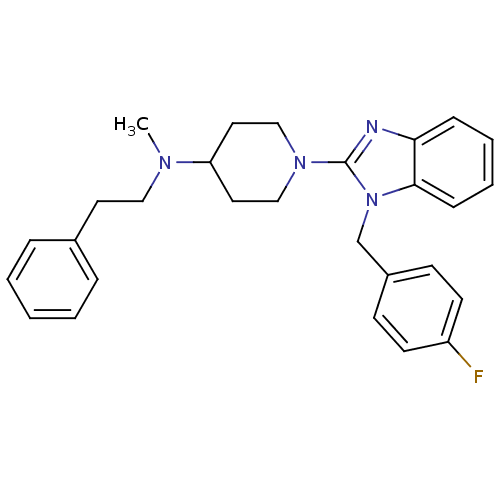
(1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...)Show SMILES CN(CCc1ccccc1)C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1 Show InChI InChI=1S/C28H31FN4/c1-31(18-15-22-7-3-2-4-8-22)25-16-19-32(20-17-25)28-30-26-9-5-6-10-27(26)33(28)21-23-11-13-24(29)14-12-23/h2-14,25H,15-21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297866
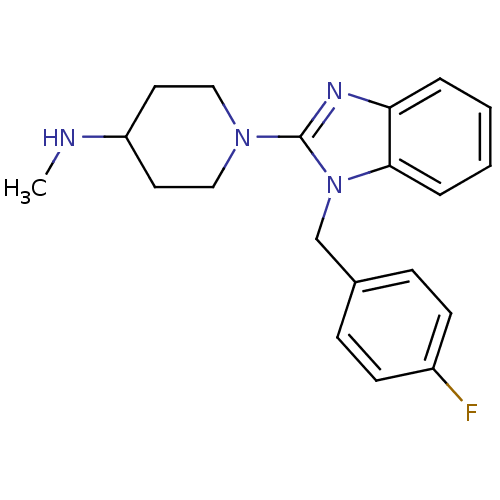
(1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...)Show InChI InChI=1S/C20H23FN4/c1-22-17-10-12-24(13-11-17)20-23-18-4-2-3-5-19(18)25(20)14-15-6-8-16(21)9-7-15/h2-9,17,22H,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50297307

(CHEMBL564226 | R-dimethindene)Show SMILES C[C@H](C1=C(CCN(C)C)Cc2ccccc12)c1ccccn1 |r,c:2| Show InChI InChI=1S/C20H24N2/c1-15(19-10-6-7-12-21-19)20-17(11-13-22(2)3)14-16-8-4-5-9-18(16)20/h4-10,12,15H,11,13-14H2,1-3H3/t15-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from human muscarinic M1 receptor expressed in CHO Flp-In cells by liquid scintillation counting |
J Med Chem 52: 5307-10 (2009)
Article DOI: 10.1021/jm900933k
BindingDB Entry DOI: 10.7270/Q2057G0S |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315208

((R)-2-(6-methoxy-3-(1-(thiazol-2-yl)ethyl)-1H-inde...)Show SMILES COc1ccc2C([C@@H](C)c3nccs3)=C(CCN(C)C)Cc2c1 |r,t:14| Show InChI InChI=1S/C19H24N2OS/c1-13(19-20-8-10-23-19)18-14(7-9-21(2)3)11-15-12-16(22-4)5-6-17(15)18/h5-6,8,10,12-13H,7,9,11H2,1-4H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22877
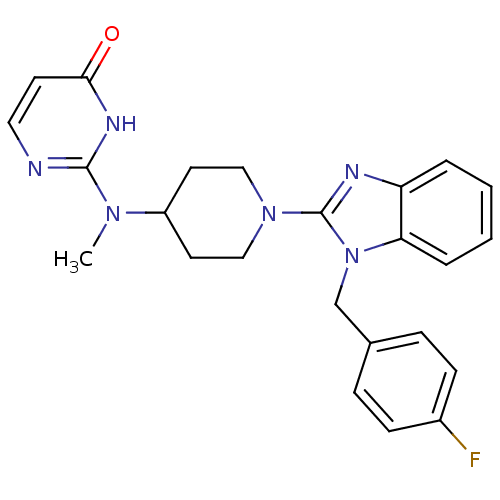
(2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...)Show SMILES CN(C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1)c1nccc(=O)[nH]1 Show InChI InChI=1S/C24H25FN6O/c1-29(23-26-13-10-22(32)28-23)19-11-14-30(15-12-19)24-27-20-4-2-3-5-21(20)31(24)16-17-6-8-18(25)9-7-17/h2-10,13,19H,11-12,14-16H2,1H3,(H,26,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315203

((R)-2-(3-(1-(3-methoxypyrazin-2-yl)ethyl)-6-methyl...)Show SMILES COc1nccnc1[C@H](C)C1=C(CCN(C)C)Cc2cc(C)ccc12 |r,c:11| Show InChI InChI=1S/C21H27N3O/c1-14-6-7-18-17(12-14)13-16(8-11-24(3)4)19(18)15(2)20-21(25-5)23-10-9-22-20/h6-7,9-10,12,15H,8,11,13H2,1-5H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315203

((R)-2-(3-(1-(3-methoxypyrazin-2-yl)ethyl)-6-methyl...)Show SMILES COc1nccnc1[C@H](C)C1=C(CCN(C)C)Cc2cc(C)ccc12 |r,c:11| Show InChI InChI=1S/C21H27N3O/c1-14-6-7-18-17(12-14)13-16(8-11-24(3)4)19(18)15(2)20-21(25-5)23-10-9-22-20/h6-7,9-10,12,15H,8,11,13H2,1-5H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of histamine H1 receptor |
Bioorg Med Chem Lett 20: 5874-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.117
BindingDB Entry DOI: 10.7270/Q26W9BDC |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297867
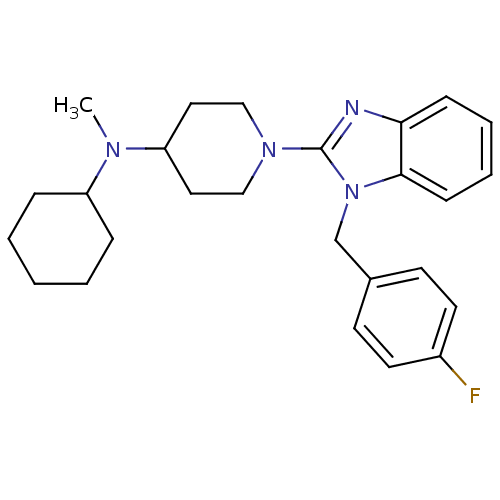
(CHEMBL558933 | N-cyclohexyl-1-(1-(4-fluorobenzyl)-...)Show SMILES CN(C1CCCCC1)C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1 Show InChI InChI=1S/C26H33FN4/c1-29(22-7-3-2-4-8-22)23-15-17-30(18-16-23)26-28-24-9-5-6-10-25(24)31(26)19-20-11-13-21(27)14-12-20/h5-6,9-14,22-23H,2-4,7-8,15-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50336074
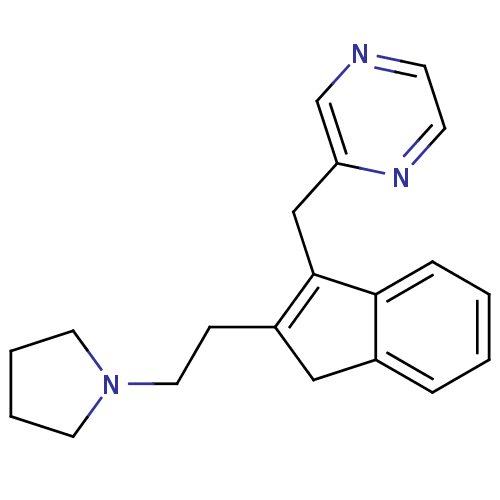
(2-((2-(2-(pyrrolidin-1-yl)ethyl)-1H-inden-3-yl)met...)Show InChI InChI=1S/C20H23N3/c1-2-6-19-16(5-1)13-17(7-12-23-10-3-4-11-23)20(19)14-18-15-21-8-9-22-18/h1-2,5-6,8-9,15H,3-4,7,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to histamine H1 receptor |
Bioorg Med Chem Lett 21: 947-51 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.053
BindingDB Entry DOI: 10.7270/Q2319W59 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297855
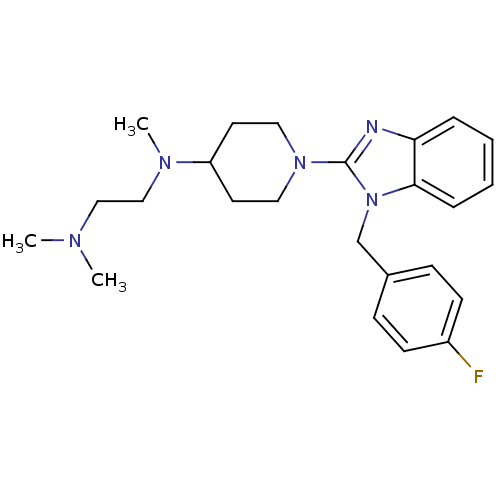
(CHEMBL559061 | N1-(1-(1-(4-fluorobenzyl)-1H-benzo[...)Show SMILES CN(C)CCN(C)C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1 Show InChI InChI=1S/C24H32FN5/c1-27(2)16-17-28(3)21-12-14-29(15-13-21)24-26-22-6-4-5-7-23(22)30(24)18-19-8-10-20(25)11-9-19/h4-11,21H,12-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315202
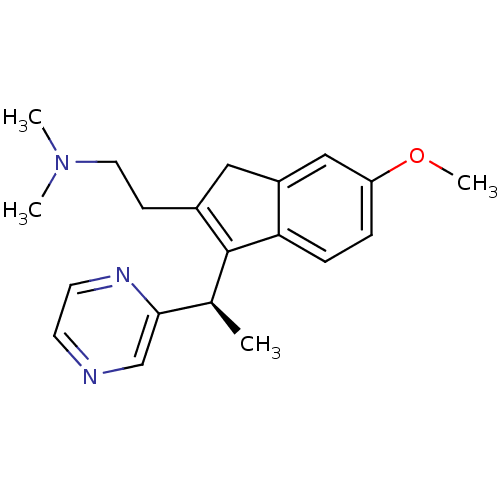
((R)-2-(6-methoxy-3-(1-(pyrazin-2-yl)ethyl)-1H-inde...)Show SMILES COc1ccc2C([C@@H](C)c3cnccn3)=C(CCN(C)C)Cc2c1 |r,t:15| Show InChI InChI=1S/C20H25N3O/c1-14(19-13-21-8-9-22-19)20-15(7-10-23(2)3)11-16-12-17(24-4)5-6-18(16)20/h5-6,8-9,12-14H,7,10-11H2,1-4H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315192
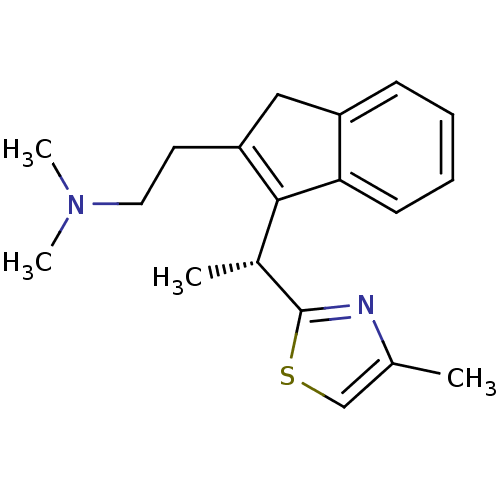
((R)-N,N-dimethyl-2-(3-(1-(4-methylthiazol-2-yl)eth...)Show SMILES C[C@H](C1=C(CCN(C)C)Cc2ccccc12)c1nc(C)cs1 |r,c:2| Show InChI InChI=1S/C19H24N2S/c1-13-12-22-19(20-13)14(2)18-16(9-10-21(3)4)11-15-7-5-6-8-17(15)18/h5-8,12,14H,9-11H2,1-4H3/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50074501
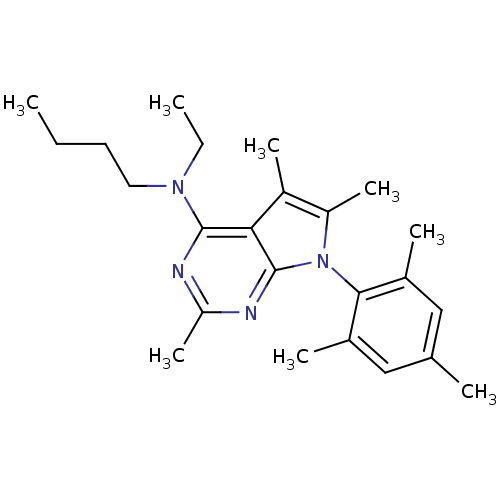
(Butyl-ethyl-[2,5,6-trimethyl-7-(2,4,6-trimethyl-ph...)Show SMILES CCCCN(CC)c1nc(C)nc2n(c(C)c(C)c12)-c1c(C)cc(C)cc1C |(4.9,1.82,;3.57,2.56,;2.24,1.79,;.91,2.55,;-.44,1.78,;-1.76,2.55,;-3.1,1.78,;-.42,.25,;-1.75,-.52,;-1.75,-2.06,;-3.08,-2.83,;-.42,-2.83,;.91,-2.06,;2.38,-2.54,;3.31,-1.29,;4.85,-1.29,;2.38,-.03,;2.86,1.43,;.91,-.52,;2.86,-4.02,;4.37,-4.33,;5.39,-3.17,;4.85,-5.78,;3.82,-6.93,;4.31,-8.4,;2.32,-6.61,;1.84,-5.14,;.33,-4.82,)| Show InChI InChI=1S/C24H34N4/c1-9-11-12-27(10-2)23-21-18(6)19(7)28(24(21)26-20(8)25-23)22-16(4)13-15(3)14-17(22)5/h13-14H,9-12H2,1-8H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for corticotropin-releasing factor-1 expressed in leukocyte tyrosine kinase cells |
J Med Chem 48: 5104-7 (2005)
Article DOI: 10.1021/jm050384+
BindingDB Entry DOI: 10.7270/Q2610ZVM |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297858
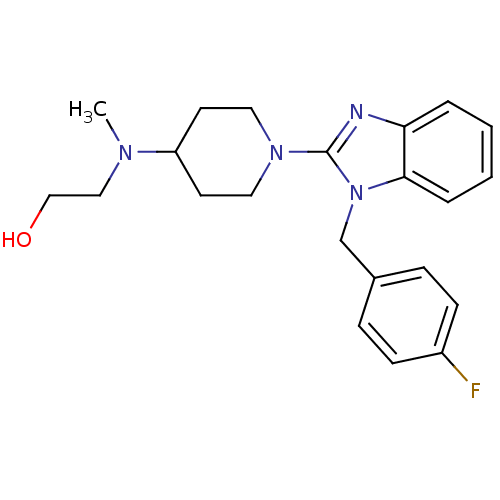
(2-((1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl...)Show InChI InChI=1S/C22H27FN4O/c1-25(14-15-28)19-10-12-26(13-11-19)22-24-20-4-2-3-5-21(20)27(22)16-17-6-8-18(23)9-7-17/h2-9,19,28H,10-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297870
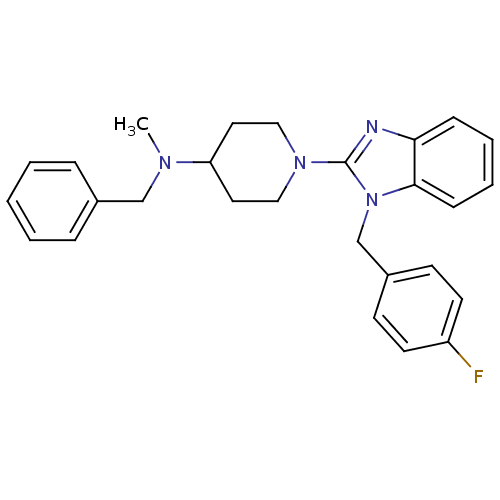
(CHEMBL551888 | N-benzyl-1-(1-(4-fluorobenzyl)-1H-b...)Show SMILES CN(Cc1ccccc1)C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1 Show InChI InChI=1S/C27H29FN4/c1-30(19-21-7-3-2-4-8-21)24-15-17-31(18-16-24)27-29-25-9-5-6-10-26(25)32(27)20-22-11-13-23(28)14-12-22/h2-14,24H,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297861
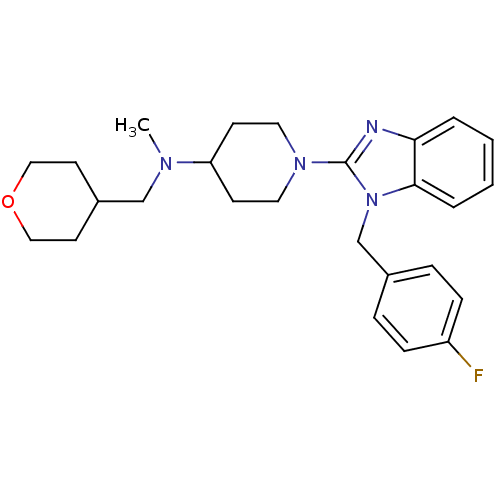
(1-(1-(4-fluorobenzyl)-1H-benzo[d]imidazol-2-yl)-N-...)Show SMILES CN(CC1CCOCC1)C1CCN(CC1)c1nc2ccccc2n1Cc1ccc(F)cc1 Show InChI InChI=1S/C26H33FN4O/c1-29(18-21-12-16-32-17-13-21)23-10-14-30(15-11-23)26-28-24-4-2-3-5-25(24)31(26)19-20-6-8-22(27)9-7-20/h2-9,21,23H,10-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation assay |
Bioorg Med Chem Lett 19: 4380-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.086
BindingDB Entry DOI: 10.7270/Q2KS6RM8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data