| Reaction Details |
|---|
 | Report a problem with these data |
| Target | C-X-C chemokine receptor type 3 |
|---|
| Ligand | BDBM50337212 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_726362 (CHEMBL1687494) |
|---|
| IC50 | 5±n/a nM |
|---|
| Citation |  Shao, Y; Anilkumar, GN; Carroll, CD; Dong, G; Hall, JW; Hobbs, DW; Jiang, Y; Jenh, CH; Kim, SH; Kozlowski, JA; McGuinness, BF; Rosenblum, SB; Schulman, I; Shih, NY; Shu, Y; Wong, MK; Yu, W; Zawacki, LG; Zeng, Q II. SAR studies of pyridyl-piperazinyl-piperidine derivatives as CXCR3 chemokine antagonists. Bioorg Med Chem Lett21:1527-31 (2011) [PubMed] Article Shao, Y; Anilkumar, GN; Carroll, CD; Dong, G; Hall, JW; Hobbs, DW; Jiang, Y; Jenh, CH; Kim, SH; Kozlowski, JA; McGuinness, BF; Rosenblum, SB; Schulman, I; Shih, NY; Shu, Y; Wong, MK; Yu, W; Zawacki, LG; Zeng, Q II. SAR studies of pyridyl-piperazinyl-piperidine derivatives as CXCR3 chemokine antagonists. Bioorg Med Chem Lett21:1527-31 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| C-X-C chemokine receptor type 3 |
|---|
| Name: | C-X-C chemokine receptor type 3 |
|---|
| Synonyms: | AAO92295.1 | C-X-C chemokine receptor type 3 | C-X-C chemokine receptor type 3 (CXCR3) | C-X-C chemokine receptor type 3 (CXCR3A) | CXCR3 | CXCR3A | CXCR3_HUMAN | GPR9 | chemokine (C-X-C motif) receptor 3 |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 40665.65 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 368 |
|---|
| Sequence: | MVLEVSDHQVLNDAEVAALLENFSSSYDYGENESDSCCTSPPCPQDFSLNFDRAFLPALY
SLLFLLGLLGNGAVAAVLLSRRTALSSTDTFLLHLAVADTLLVLTLPLWAVDAAVQWVFG
SGLCKVAGALFNINFYAGALLLACISFDRYLNIVHATQLYRRGPPARVTLTCLAVWGLCL
LFALPDFIFLSAHHDERLNATHCQYNFPQVGRTALRVLQLVAGFLLPLLVMAYCYAHILA
VLLVSRGQRRLRAMRLVVVVVVAFALCWTPYHLVVLVDILMDLGALARNCGRESRVDVAK
SVTSGLGYMHCCLNPLLYAFVGVKFRERMWMLLLRLGCPNQRGLQRQPSSSRRDSSWSET
SEASYSGL
|
|
|
|---|
| BDBM50337212 |
|---|
| n/a |
|---|
| Name | BDBM50337212 |
|---|
| Synonyms: | 5-chloro-6-((3S)-4-(1-(1-(4-chlorophenyl)ethyl)piperidin-4-yl)-3-methylpiperazin-1-yl)-N-methylnicotinamide | CHEMBL1681832 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H33Cl2N5O |
|---|
| Mol. Mass. | 490.468 |
|---|
| SMILES | CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(CC2)C(C)c2ccc(Cl)cc2)c(Cl)c1 |r| |
|---|
| Structure | 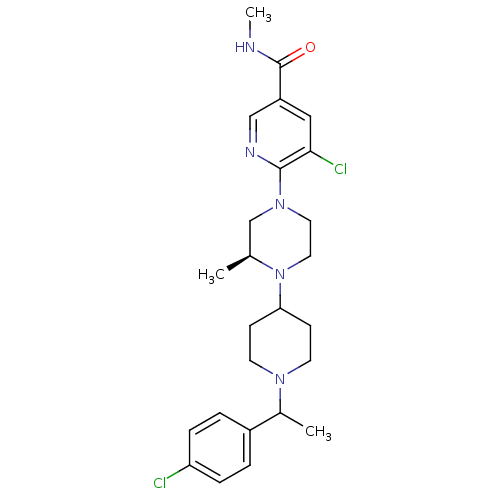
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Shao, Y; Anilkumar, GN; Carroll, CD; Dong, G; Hall, JW; Hobbs, DW; Jiang, Y; Jenh, CH; Kim, SH; Kozlowski, JA; McGuinness, BF; Rosenblum, SB; Schulman, I; Shih, NY; Shu, Y; Wong, MK; Yu, W; Zawacki, LG; Zeng, Q II. SAR studies of pyridyl-piperazinyl-piperidine derivatives as CXCR3 chemokine antagonists. Bioorg Med Chem Lett21:1527-31 (2011) [PubMed] Article
Shao, Y; Anilkumar, GN; Carroll, CD; Dong, G; Hall, JW; Hobbs, DW; Jiang, Y; Jenh, CH; Kim, SH; Kozlowski, JA; McGuinness, BF; Rosenblum, SB; Schulman, I; Shih, NY; Shu, Y; Wong, MK; Yu, W; Zawacki, LG; Zeng, Q II. SAR studies of pyridyl-piperazinyl-piperidine derivatives as CXCR3 chemokine antagonists. Bioorg Med Chem Lett21:1527-31 (2011) [PubMed] Article