 Found 34345 hits with Last Name = 'jiang' and Initial = 'y'
Found 34345 hits with Last Name = 'jiang' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50607169
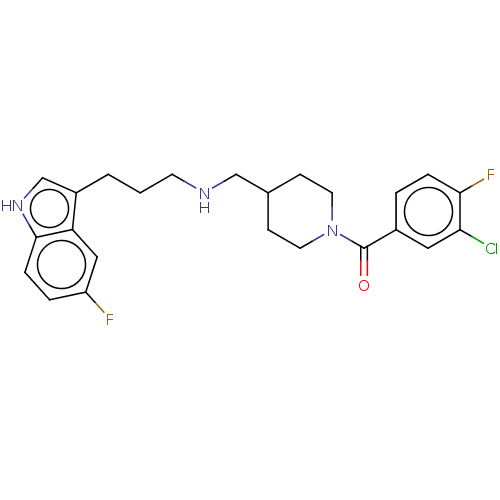
(CHEMBL5220371)Show SMILES Fc1ccc2[nH]cc(CCCNCC3CCN(CC3)C(=O)c3ccc(F)c(Cl)c3)c2c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.129006
BindingDB Entry DOI: 10.7270/Q2ZC8701 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50607170
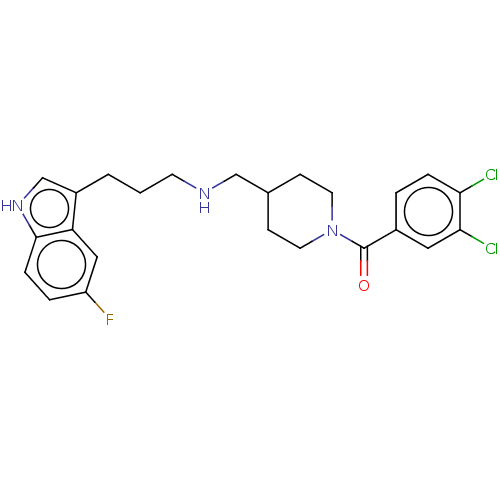
(CHEMBL5220872)Show SMILES Fc1ccc2[nH]cc(CCCNCC3CCN(CC3)C(=O)c3ccc(Cl)c(Cl)c3)c2c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.129006
BindingDB Entry DOI: 10.7270/Q2ZC8701 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50428107
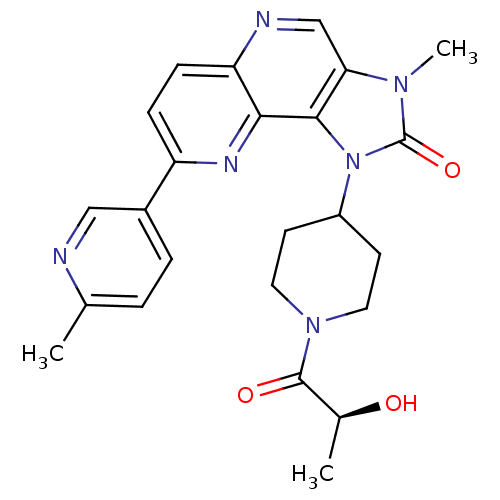
(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50428107

(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kdelta |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50428107

(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428109
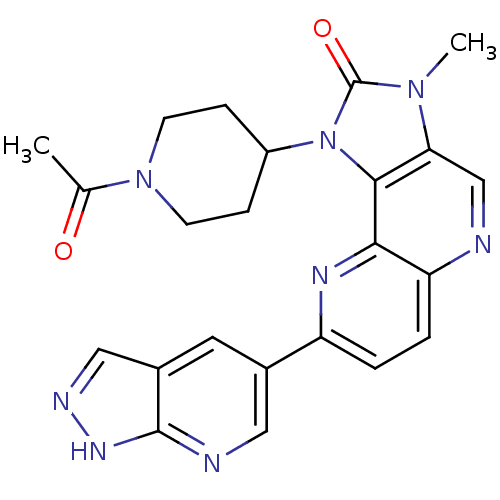
(CHEMBL2331668 | US8791131, 259)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C23H22N8O2/c1-13(32)30-7-5-16(6-8-30)31-21-19(29(2)23(31)33)12-24-18-4-3-17(27-20(18)21)14-9-15-11-26-28-22(15)25-10-14/h3-4,9-12,16H,5-8H2,1-2H3,(H,25,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50529312

(CHEMBL4444213)Show SMILES Fc1ccc(OCCN2CCC(CC2)c2c[nH]c3ccc(F)cc23)c(c1)-c1ccccc1 Show InChI InChI=1S/C27H26F2N2O/c28-21-6-8-26-24(17-21)25(18-30-26)20-10-12-31(13-11-20)14-15-32-27-9-7-22(29)16-23(27)19-4-2-1-3-5-19/h1-9,16-18,20,30H,10-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.129006
BindingDB Entry DOI: 10.7270/Q2ZC8701 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428109

(CHEMBL2331668 | US8791131, 259)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C23H22N8O2/c1-13(32)30-7-5-16(6-8-30)31-21-19(29(2)23(31)33)12-24-18-4-3-17(27-20(18)21)14-9-15-11-26-28-22(15)25-10-14/h3-4,9-12,16H,5-8H2,1-2H3,(H,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50152456
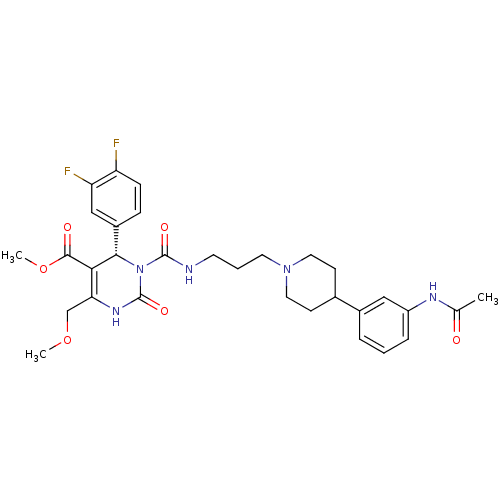
((S)-3-{3-[4-(3-Acetylamino-phenyl)-piperidin-1-yl]...)Show SMILES COCC1=C([C@@H](N(C(=O)NCCCN2CCC(CC2)c2cccc(NC(C)=O)c2)C(=O)N1)c1ccc(F)c(F)c1)C(=O)OC |t:3| Show InChI InChI=1S/C31H37F2N5O6/c1-19(39)35-23-7-4-6-21(16-23)20-10-14-37(15-11-20)13-5-12-34-30(41)38-28(22-8-9-24(32)25(33)17-22)27(29(40)44-3)26(18-43-2)36-31(38)42/h4,6-9,16-17,20,28H,5,10-15,18H2,1-3H3,(H,34,41)(H,35,39)(H,36,42)/t28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]T226296 from rat recombinant MCH1 receptor |
J Med Chem 50: 3883-90 (2007)
Article DOI: 10.1021/jm060383x
BindingDB Entry DOI: 10.7270/Q25D8RJM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50152456

((S)-3-{3-[4-(3-Acetylamino-phenyl)-piperidin-1-yl]...)Show SMILES COCC1=C([C@@H](N(C(=O)NCCCN2CCC(CC2)c2cccc(NC(C)=O)c2)C(=O)N1)c1ccc(F)c(F)c1)C(=O)OC |t:3| Show InChI InChI=1S/C31H37F2N5O6/c1-19(39)35-23-7-4-6-21(16-23)20-10-14-37(15-11-20)13-5-12-34-30(41)38-28(22-8-9-24(32)25(33)17-22)27(29(40)44-3)26(18-43-2)36-31(38)42/h4,6-9,16-17,20,28H,5,10-15,18H2,1-3H3,(H,34,41)(H,35,39)(H,36,42)/t28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428107

(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.299 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428111
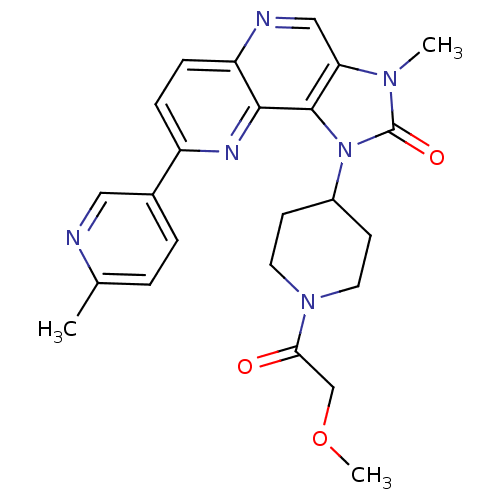
(CHEMBL2331666 | US8791131, 153)Show SMILES COCC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O Show InChI InChI=1S/C24H26N6O3/c1-15-4-5-16(12-25-15)18-6-7-19-22(27-18)23-20(13-26-19)28(2)24(32)30(23)17-8-10-29(11-9-17)21(31)14-33-3/h4-7,12-13,17H,8-11,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428108
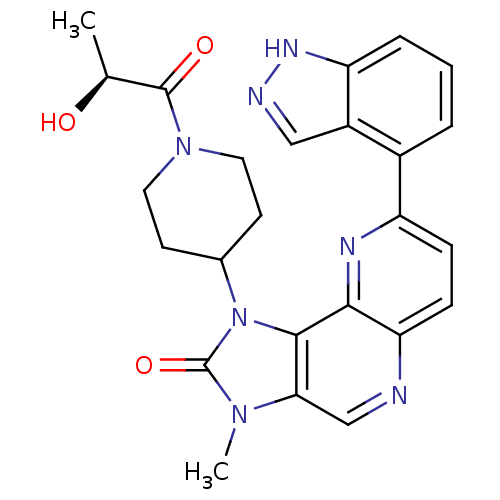
(CHEMBL2331669 | US8791131, 255)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cccc3[nH]ncc23)n(C)c1=O |r| Show InChI InChI=1S/C25H25N7O3/c1-14(33)24(34)31-10-8-15(9-11-31)32-23-21(30(2)25(32)35)13-26-20-7-6-18(28-22(20)23)16-4-3-5-19-17(16)12-27-29-19/h3-7,12-15,33H,8-11H2,1-2H3,(H,27,29)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.395 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50331916
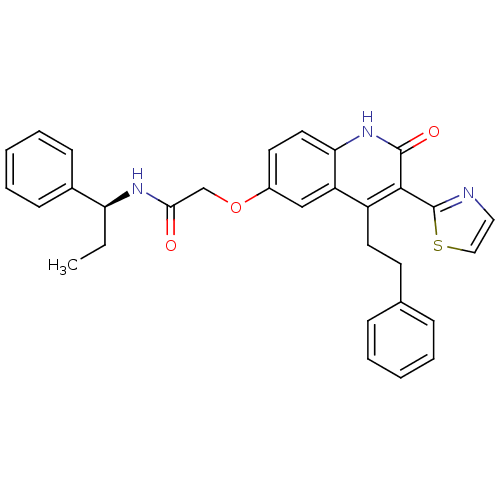
((S)-2-(2-oxo-4-phenethyl-3-(thiazol-2-yl)-1,2-dihy...)Show SMILES CC[C@H](NC(=O)COc1ccc2[nH]c(=O)c(-c3nccs3)c(CCc3ccccc3)c2c1)c1ccccc1 |r| Show InChI InChI=1S/C31H29N3O3S/c1-2-26(22-11-7-4-8-12-22)33-28(35)20-37-23-14-16-27-25(19-23)24(15-13-21-9-5-3-6-10-21)29(30(36)34-27)31-32-17-18-38-31/h3-12,14,16-19,26H,2,13,15,20H2,1H3,(H,33,35)(H,34,36)/t26-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosien A2B receptor in human HMC-1 cells assessed as inhibition of NECA-induced IL-8 release after 6 hr by ELISA |
Bioorg Med Chem Lett 20: 7414-20 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.030
BindingDB Entry DOI: 10.7270/Q2WH2Q60 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50331917
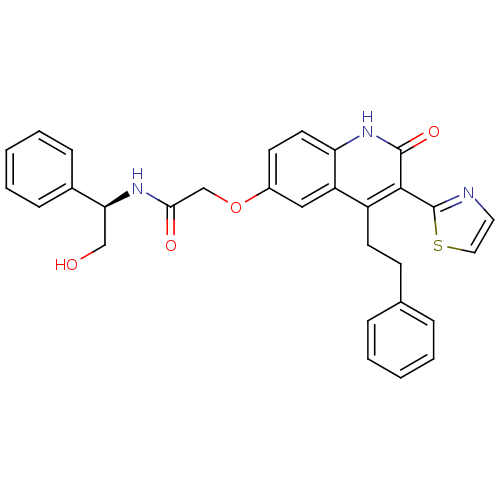
((R)-N-(2-hydroxy-1-phenylethyl)-2-(2-oxo-4-pheneth...)Show SMILES OC[C@H](NC(=O)COc1ccc2[nH]c(=O)c(-c3nccs3)c(CCc3ccccc3)c2c1)c1ccccc1 |r| Show InChI InChI=1S/C30H27N3O4S/c34-18-26(21-9-5-2-6-10-21)32-27(35)19-37-22-12-14-25-24(17-22)23(13-11-20-7-3-1-4-8-20)28(29(36)33-25)30-31-15-16-38-30/h1-10,12,14-17,26,34H,11,13,18-19H2,(H,32,35)(H,33,36)/t26-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosien A2B receptor in human HMC-1 cells assessed as inhibition of NECA-induced IL-8 release after 6 hr by ELISA |
Bioorg Med Chem Lett 20: 7414-20 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.030
BindingDB Entry DOI: 10.7270/Q2WH2Q60 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50441652
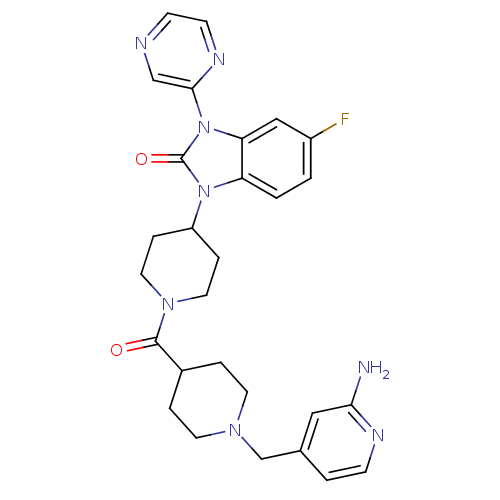
(CHEMBL2437433)Show SMILES Nc1cc(CN2CCC(CC2)C(=O)N2CCC(CC2)n2c3ccc(F)cc3n(-c3cnccn3)c2=O)ccn1 Show InChI InChI=1S/C28H31FN8O2/c29-21-1-2-23-24(16-21)37(26-17-31-9-10-33-26)28(39)36(23)22-6-13-35(14-7-22)27(38)20-4-11-34(12-5-20)18-19-3-8-32-25(30)15-19/h1-3,8-10,15-17,20,22H,4-7,11-14,18H2,(H2,30,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenates |
Bioorg Med Chem Lett 23: 6001-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.012
BindingDB Entry DOI: 10.7270/Q2542Q1F |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428113
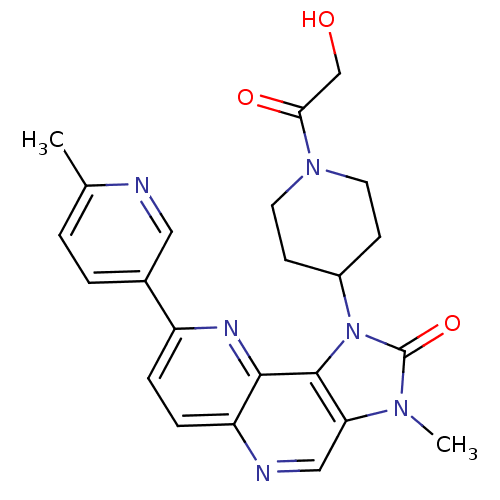
(CHEMBL2331663 | US8791131, 172)Show SMILES Cc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)CO)c3c2n1 Show InChI InChI=1S/C23H24N6O3/c1-14-3-4-15(11-24-14)17-5-6-18-21(26-17)22-19(12-25-18)27(2)23(32)29(22)16-7-9-28(10-8-16)20(31)13-30/h3-6,11-12,16,30H,7-10,13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.532 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428115
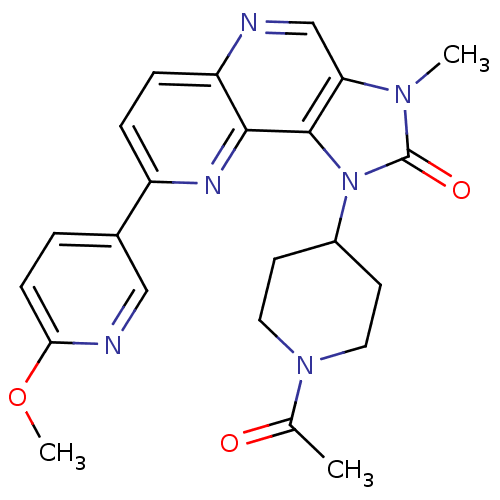
(CHEMBL2331661 | US8791131, 136)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(C)=O)c3c2n1 Show InChI InChI=1S/C23H24N6O3/c1-14(30)28-10-8-16(9-11-28)29-22-19(27(2)23(29)31)13-24-18-6-5-17(26-21(18)22)15-4-7-20(32-3)25-12-15/h4-7,12-13,16H,8-11H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.542 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428110
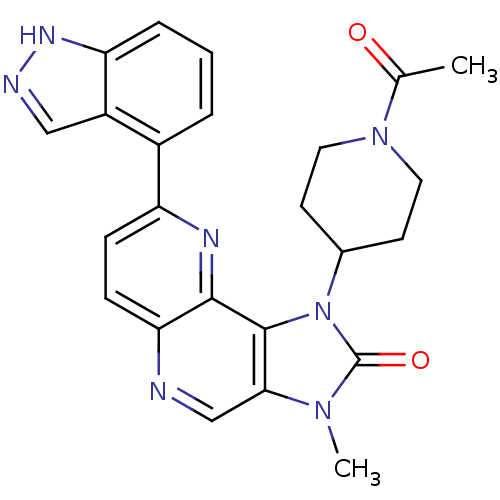
(CHEMBL2331667 | US8791131, 254)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cccc3[nH]ncc23)n(C)c1=O Show InChI InChI=1S/C24H23N7O2/c1-14(32)30-10-8-15(9-11-30)31-23-21(29(2)24(31)33)13-25-20-7-6-18(27-22(20)23)16-4-3-5-19-17(16)12-26-28-19/h3-7,12-13,15H,8-11H2,1-2H3,(H,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.584 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50441649
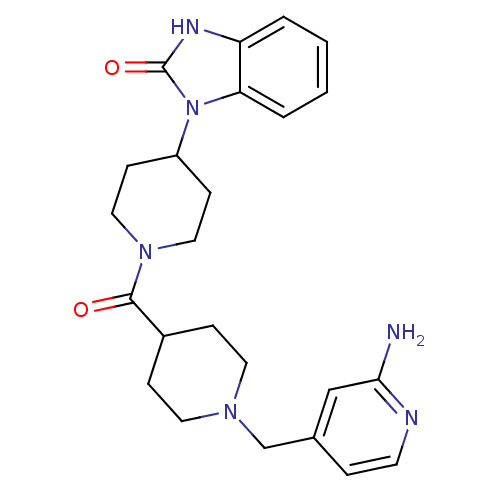
(CHEMBL2437416)Show SMILES Nc1cc(CN2CCC(CC2)C(=O)N2CCC(CC2)n2c3ccccc3[nH]c2=O)ccn1 Show InChI InChI=1S/C24H30N6O2/c25-22-15-17(5-10-26-22)16-28-11-6-18(7-12-28)23(31)29-13-8-19(9-14-29)30-21-4-2-1-3-20(21)27-24(30)32/h1-5,10,15,18-19H,6-9,11-14,16H2,(H2,25,26)(H,27,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenates |
Bioorg Med Chem Lett 23: 6001-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.012
BindingDB Entry DOI: 10.7270/Q2542Q1F |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219036

(CHEMBL388440 | N-{3-[1-(3-{[hydroxy(diphenyl)acety...)Show SMILES CC(C)C(=O)Nc1ccc(C)c(c1)C1CCN(CCCNC(=O)C(O)(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C33H41N3O3/c1-24(2)31(37)35-29-16-15-25(3)30(23-29)26-17-21-36(22-18-26)20-10-19-34-32(38)33(39,27-11-6-4-7-12-27)28-13-8-5-9-14-28/h4-9,11-16,23-24,26,39H,10,17-22H2,1-3H3,(H,34,38)(H,35,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM289804

(Methyl (4-(((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2...)Show SMILES COC(=O)Nc1ccc(cc1)C(=O)NC(CC1(CC1)OC)C(=O)N1CCC[C@@]2(C1)OC(=O)Nc1ccc(Cl)c(F)c21 |r| Show InChI InChI=1S/C28H30ClFN4O7/c1-39-25(37)31-17-6-4-16(5-7-17)23(35)32-20(14-27(40-2)11-12-27)24(36)34-13-3-10-28(15-34)21-19(33-26(38)41-28)9-8-18(29)22(21)30/h4-9,20H,3,10-15H2,1-2H3,(H,31,37)(H,32,35)(H,33,38)/t20?,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... |
US Patent US10093683 (2018)
BindingDB Entry DOI: 10.7270/Q2RR2197 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219040

(CHEMBL429464 | N-{3-[1-(3-{[bis(4-fluorophenyl)ace...)Show SMILES CC(C)C(=O)Nc1ccc(C)c(c1)C1CCN(CCCNC(=O)C(c2ccc(F)cc2)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C33H39F2N3O2/c1-22(2)32(39)37-29-14-5-23(3)30(21-29)24-15-19-38(20-16-24)18-4-17-36-33(40)31(25-6-10-27(34)11-7-25)26-8-12-28(35)13-9-26/h5-14,21-22,24,31H,4,15-20H2,1-3H3,(H,36,40)(H,37,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM289851
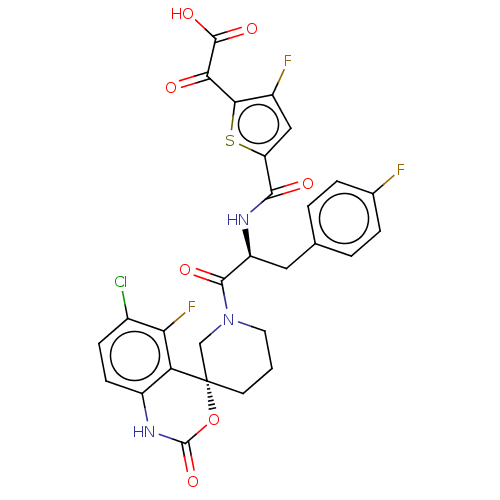
(2-(5-(((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2-dihy...)Show SMILES OC(=O)C(=O)c1sc(cc1F)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC[C@@]2(C1)OC(=O)Nc1ccc(Cl)c(F)c21 |r| Show InChI InChI=1S/C28H21ClF3N3O7S/c29-15-6-7-17-20(21(15)32)28(42-27(41)34-17)8-1-9-35(12-28)25(38)18(10-13-2-4-14(30)5-3-13)33-24(37)19-11-16(31)23(43-19)22(36)26(39)40/h2-7,11,18H,1,8-10,12H2,(H,33,37)(H,34,41)(H,39,40)/t18-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... |
US Patent US10093683 (2018)
BindingDB Entry DOI: 10.7270/Q2RR2197 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50088373
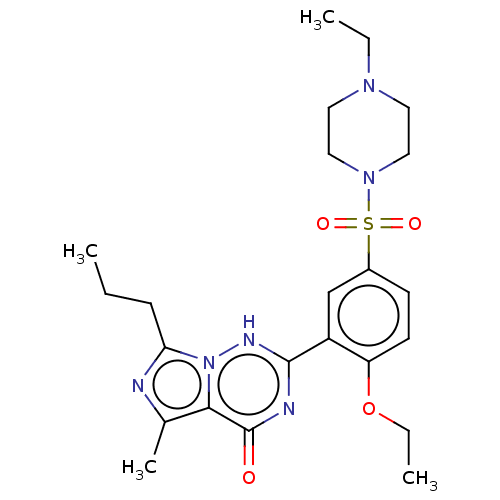
(CHEBI:46295 | Vardenafil | cid_110634)Show SMILES CCCc1nc(C)c2n1[nH]c(nc2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H32N6O4S/c1-5-8-20-24-16(4)21-23(30)25-22(26-29(20)21)18-15-17(9-10-19(18)33-7-3)34(31,32)28-13-11-27(6-2)12-14-28/h9-10,15H,5-8,11-14H2,1-4H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) |
Eur J Med Chem 158: 767-780 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.028
BindingDB Entry DOI: 10.7270/Q2JS9T4N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(GUINEA PIG) | BDBM50441646
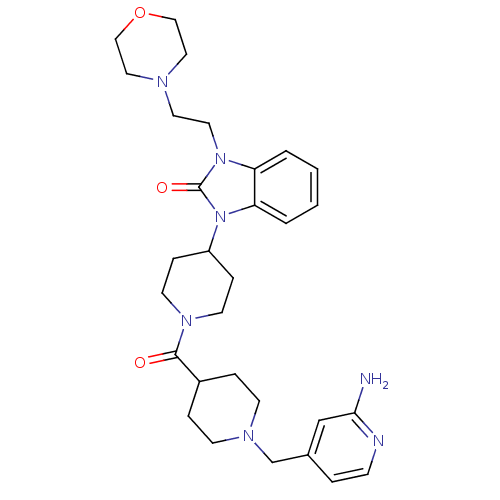
(CHEMBL2437421)Show SMILES Nc1cc(CN2CCC(CC2)C(=O)N2CCC(CC2)n2c3ccccc3n(CCN3CCOCC3)c2=O)ccn1 Show InChI InChI=1S/C30H41N7O3/c31-28-21-23(5-10-32-28)22-34-11-6-24(7-12-34)29(38)35-13-8-25(9-14-35)37-27-4-2-1-3-26(27)36(30(37)39)16-15-33-17-19-40-20-18-33/h1-5,10,21,24-25H,6-9,11-20,22H2,(H2,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenates |
Bioorg Med Chem Lett 23: 6001-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.012
BindingDB Entry DOI: 10.7270/Q2542Q1F |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM289807
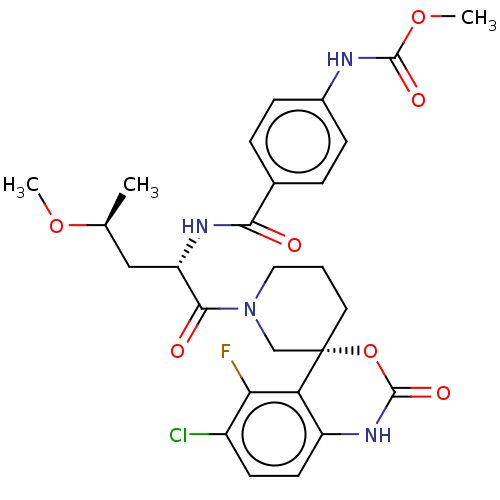
(US10093683, Example 118A | US10093683, Example 118...)Show SMILES CO[C@@H](C)C[C@H](NC(=O)c1ccc(NC(=O)OC)cc1)C(=O)N1CCC[C@@]2(C1)OC(=O)Nc1ccc(Cl)c(F)c21 |r| Show InChI InChI=1S/C27H30ClFN4O7/c1-15(38-2)13-20(31-23(34)16-5-7-17(8-6-16)30-25(36)39-3)24(35)33-12-4-11-27(14-33)21-19(32-26(37)40-27)10-9-18(28)22(21)29/h5-10,15,20H,4,11-14H2,1-3H3,(H,30,36)(H,31,34)(H,32,37)/t15-,20-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.720 | -52.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... |
US Patent US10093683 (2018)
BindingDB Entry DOI: 10.7270/Q2RR2197 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428113

(CHEMBL2331663 | US8791131, 172)Show SMILES Cc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)CO)c3c2n1 Show InChI InChI=1S/C23H24N6O3/c1-14-3-4-15(11-24-14)17-5-6-18-21(26-17)22-19(12-25-18)27(2)23(32)29(22)16-7-9-28(10-8-16)20(31)13-30/h3-6,11-12,16,30H,7-10,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.842 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428111

(CHEMBL2331666 | US8791131, 153)Show SMILES COCC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O Show InChI InChI=1S/C24H26N6O3/c1-15-4-5-16(12-25-15)18-6-7-19-22(27-18)23-20(13-26-19)28(2)24(32)30(23)17-8-10-29(11-9-17)21(31)14-33-3/h4-7,12-13,17H,8-11,14H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.922 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50441637
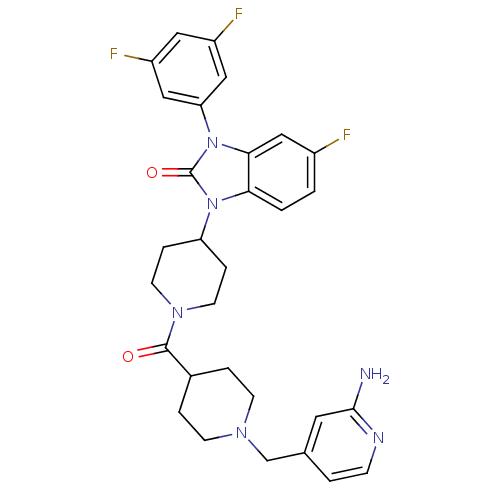
(CHEMBL2437430)Show SMILES Nc1cc(CN2CCC(CC2)C(=O)N2CCC(CC2)n2c3ccc(F)cc3n(-c3cc(F)cc(F)c3)c2=O)ccn1 Show InChI InChI=1S/C30H31F3N6O2/c31-21-1-2-26-27(17-21)39(25-15-22(32)14-23(33)16-25)30(41)38(26)24-6-11-37(12-7-24)29(40)20-4-9-36(10-5-20)18-19-3-8-35-28(34)13-19/h1-3,8,13-17,20,24H,4-7,9-12,18H2,(H2,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenates |
Bioorg Med Chem Lett 23: 6001-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.012
BindingDB Entry DOI: 10.7270/Q2542Q1F |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM333146
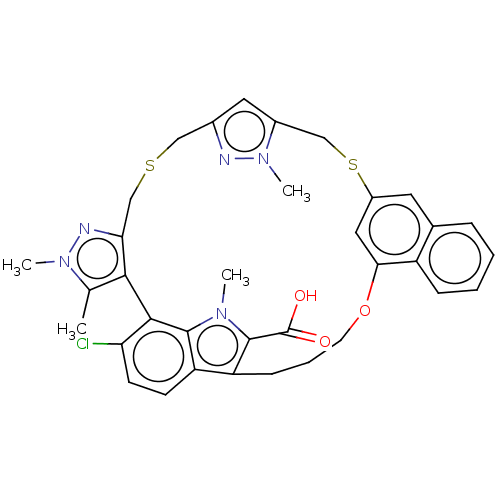
(Compound I | US10196404, Example 1 | US10196404, E...)Show SMILES Cc1c-2c(CSCc3cc(CSc4cc(OCCCc5c(C(O)=O)n(C)c6c-2c(Cl)ccc56)c2ccccc2c4)n(C)n3)nn1C Show InChI InChI=1S/C35H34ClN5O3S2/c1-20-31-29(38-40(20)3)19-45-17-22-15-23(41(4)37-22)18-46-24-14-21-8-5-6-9-25(21)30(16-24)44-13-7-10-26-27-11-12-28(36)32(31)33(27)39(2)34(26)35(42)43/h5-6,8-9,11-12,14-16H,7,10,13,17-19H2,1-4H3,(H,42,43) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FITC-labeled Bak BH3 peptide binding to GST-tagged MCL1 (171 to 327 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3)... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00682
BindingDB Entry DOI: 10.7270/Q2MC93VV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50331914
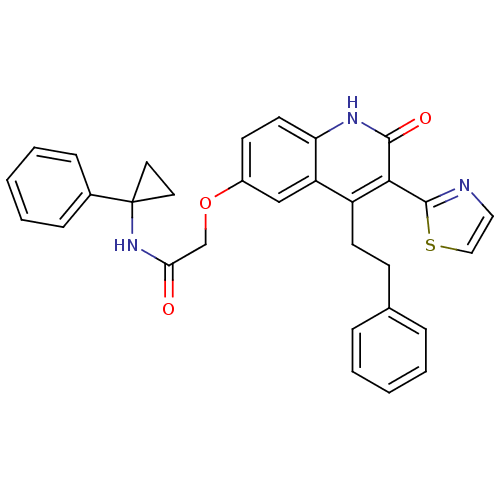
(2-(2-oxo-4-phenethyl-3-(thiazol-2-yl)-1,2-dihydroq...)Show SMILES O=C(COc1ccc2[nH]c(=O)c(-c3nccs3)c(CCc3ccccc3)c2c1)NC1(CC1)c1ccccc1 Show InChI InChI=1S/C31H27N3O3S/c35-27(34-31(15-16-31)22-9-5-2-6-10-22)20-37-23-12-14-26-25(19-23)24(13-11-21-7-3-1-4-8-21)28(29(36)33-26)30-32-17-18-38-30/h1-10,12,14,17-19H,11,13,15-16,20H2,(H,33,36)(H,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosien A2B receptor in human HMC-1 cells assessed as inhibition of NECA-induced IL-8 release after 6 hr by ELISA |
Bioorg Med Chem Lett 20: 7414-20 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.030
BindingDB Entry DOI: 10.7270/Q2WH2Q60 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM24466
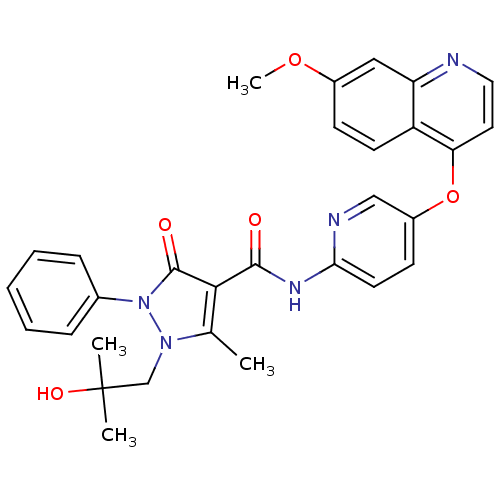
(1-(2-hydroxy-2-methylpropyl)-N-{5-[(7-methoxyquino...)Show SMILES COc1ccc2c(Oc3ccc(NC(=O)c4c(C)n(CC(C)(C)O)n(-c5ccccc5)c4=O)nc3)ccnc2c1 Show InChI InChI=1S/C30H29N5O5/c1-19-27(29(37)35(20-8-6-5-7-9-20)34(19)18-30(2,3)38)28(36)33-26-13-11-22(17-32-26)40-25-14-15-31-24-16-21(39-4)10-12-23(24)25/h5-17,38H,18H2,1-4H3,(H,32,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) |
Eur J Med Chem 108: 495-504 (2016)
Article DOI: 10.1016/j.ejmech.2015.12.016
BindingDB Entry DOI: 10.7270/Q2G73GMC |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50519614

(CHEMBL4439276)Show SMILES CN1CCN(CCOc2ccc(-c3c(sc4ncnc(O[C@H](Cc5ccccc5OCc5ccnn5CC(F)(F)F)C(O)=O)c34)-c3ccc(F)o3)c(C)c2Cl)CC1 |r,wD:21.21,(19.44,-4.89,;18.41,-6.03,;18.89,-7.5,;17.87,-8.64,;16.35,-8.32,;15.33,-9.47,;15.81,-10.94,;14.78,-12.09,;15.27,-13.55,;16.78,-13.86,;17.26,-15.32,;16.23,-16.47,;16.71,-17.93,;15.81,-19.18,;16.72,-20.42,;18.18,-19.94,;19.51,-20.71,;20.85,-19.94,;20.85,-18.39,;19.51,-17.62,;19.51,-16.08,;20.84,-15.31,;20.83,-13.77,;22.16,-13,;23.49,-13.77,;24.82,-12.99,;24.82,-11.45,;23.47,-10.69,;22.15,-11.46,;20.81,-10.71,;20.79,-9.17,;22.12,-8.38,;23.53,-9,;24.55,-7.84,;23.76,-6.51,;22.26,-6.85,;21.1,-5.83,;21.09,-4.29,;22.42,-3.51,;19.76,-3.52,;21.09,-2.75,;22.17,-16.08,;23.5,-15.3,;22.18,-17.62,;18.17,-18.4,;14.27,-19.19,;13.36,-17.95,;11.89,-18.43,;11.9,-19.97,;10.66,-20.88,;13.37,-20.44,;14.73,-16.16,;13.7,-17.31,;14.24,-14.71,;12.73,-14.4,;15.87,-6.87,;16.9,-5.72,)| Show InChI InChI=1S/C39H37ClF4N6O6S/c1-23-26(7-8-28(34(23)40)53-18-17-49-15-13-48(2)14-16-49)32-33-36(45-22-46-37(33)57-35(32)29-9-10-31(41)55-29)56-30(38(51)52)19-24-5-3-4-6-27(24)54-20-25-11-12-47-50(25)21-39(42,43)44/h3-12,22,30H,13-21H2,1-2H3,(H,51,52)/t30-/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to C-terminal MBP-fused human MCL1 (173 to 321 residues) expressed in Escherichia coli BL21(DE3)pLysS incubated for 2 hrs by fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00682
BindingDB Entry DOI: 10.7270/Q2MC93VV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50331913
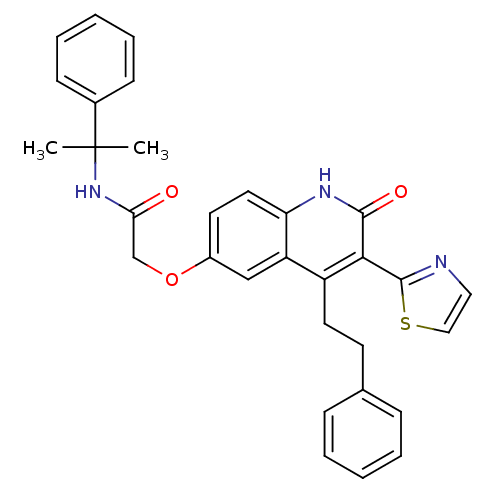
(2-(2-oxo-4-phenethyl-3-(thiazol-2-yl)-1,2-dihydroq...)Show SMILES CC(C)(NC(=O)COc1ccc2[nH]c(=O)c(-c3nccs3)c(CCc3ccccc3)c2c1)c1ccccc1 Show InChI InChI=1S/C31H29N3O3S/c1-31(2,22-11-7-4-8-12-22)34-27(35)20-37-23-14-16-26-25(19-23)24(15-13-21-9-5-3-6-10-21)28(29(36)33-26)30-32-17-18-38-30/h3-12,14,16-19H,13,15,20H2,1-2H3,(H,33,36)(H,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosien A2B receptor in human HMC-1 cells assessed as inhibition of NECA-induced IL-8 release after 6 hr by ELISA |
Bioorg Med Chem Lett 20: 7414-20 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.030
BindingDB Entry DOI: 10.7270/Q2WH2Q60 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219026
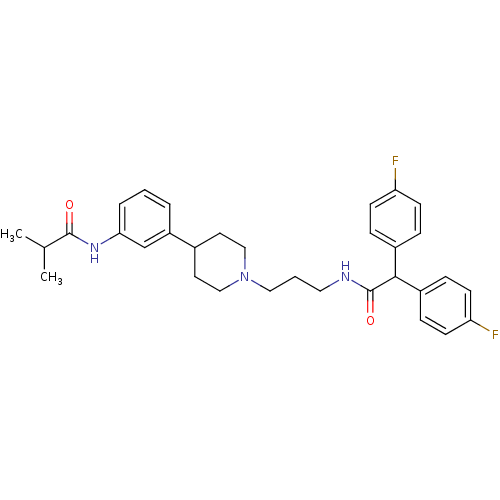
(CHEMBL243338 | N-{3-[1-(3-{[bis(4-fluorophenyl)ace...)Show SMILES CC(C)C(=O)Nc1cccc(c1)C1CCN(CCCNC(=O)C(c2ccc(F)cc2)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C32H37F2N3O2/c1-22(2)31(38)36-29-6-3-5-26(21-29)23-15-19-37(20-16-23)18-4-17-35-32(39)30(24-7-11-27(33)12-8-24)25-9-13-28(34)14-10-25/h3,5-14,21-23,30H,4,15-20H2,1-2H3,(H,35,39)(H,36,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428117
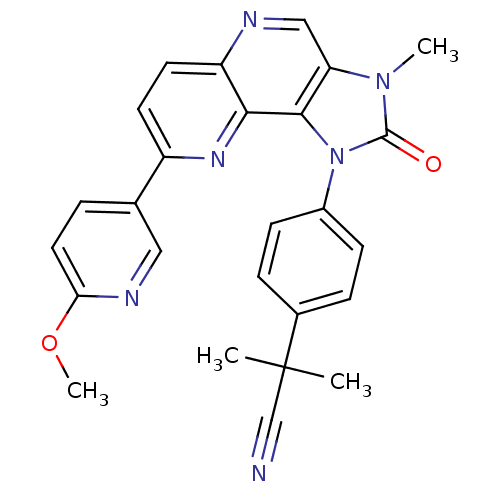
(CHEMBL2331657)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(-c4ccc(cc4)C(C)(C)C#N)c3c2n1 Show InChI InChI=1S/C26H22N6O2/c1-26(2,15-27)17-6-8-18(9-7-17)32-24-21(31(3)25(32)33)14-28-20-11-10-19(30-23(20)24)16-5-12-22(34-4)29-13-16/h5-14H,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428107

(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50441642
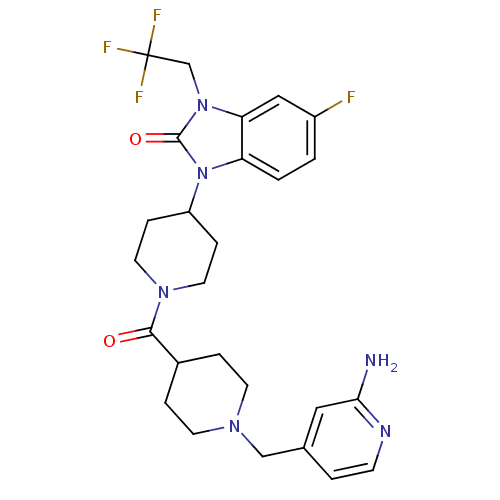
(CHEMBL2437425)Show SMILES Nc1cc(CN2CCC(CC2)C(=O)N2CCC(CC2)n2c3ccc(F)cc3n(CC(F)(F)F)c2=O)ccn1 Show InChI InChI=1S/C26H30F4N6O2/c27-19-1-2-21-22(14-19)35(16-26(28,29)30)25(38)36(21)20-6-11-34(12-7-20)24(37)18-4-9-33(10-5-18)15-17-3-8-32-23(31)13-17/h1-3,8,13-14,18,20H,4-7,9-12,15-16H2,(H2,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenates |
Bioorg Med Chem Lett 23: 6001-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.012
BindingDB Entry DOI: 10.7270/Q2542Q1F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50441647
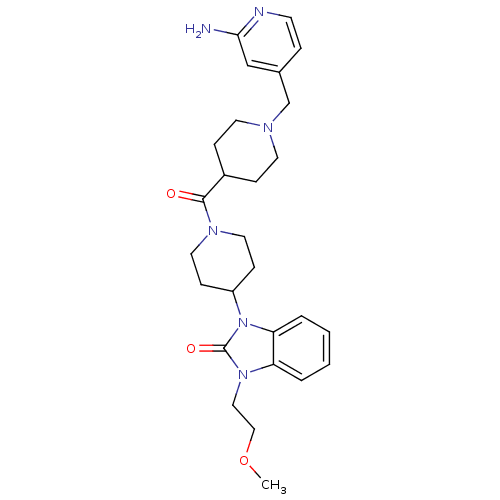
(CHEMBL2437420)Show SMILES COCCn1c2ccccc2n(C2CCN(CC2)C(=O)C2CCN(Cc3ccnc(N)c3)CC2)c1=O Show InChI InChI=1S/C27H36N6O3/c1-36-17-16-32-23-4-2-3-5-24(23)33(27(32)35)22-9-14-31(15-10-22)26(34)21-7-12-30(13-8-21)19-20-6-11-29-25(28)18-20/h2-6,11,18,21-22H,7-10,12-17,19H2,1H3,(H2,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor in guinea pig brain homogenates |
Bioorg Med Chem Lett 23: 6001-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.012
BindingDB Entry DOI: 10.7270/Q2542Q1F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50472100
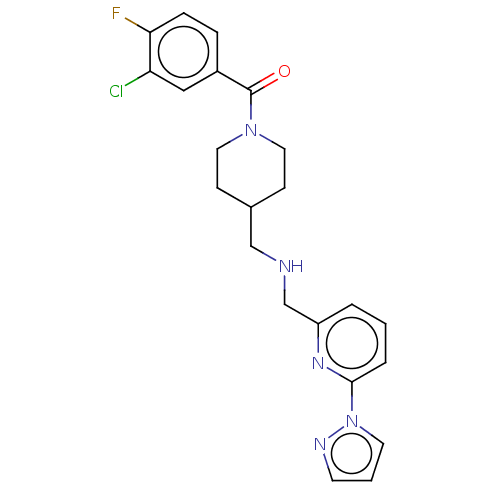
(CHEMBL147922)Show SMILES Fc1ccc(cc1Cl)C(=O)N1CCC(CNCc2cccc(n2)-n2cccn2)CC1 Show InChI InChI=1S/C22H23ClFN5O/c23-19-13-17(5-6-20(19)24)22(30)28-11-7-16(8-12-28)14-25-15-18-3-1-4-21(27-18)29-10-2-9-26-29/h1-6,9-10,13,16,25H,7-8,11-12,14-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.129006
BindingDB Entry DOI: 10.7270/Q2ZC8701 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) |
Eur J Med Chem 158: 767-780 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.028
BindingDB Entry DOI: 10.7270/Q2JS9T4N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428110

(CHEMBL2331667 | US8791131, 254)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cccc3[nH]ncc23)n(C)c1=O Show InChI InChI=1S/C24H23N7O2/c1-14(32)30-10-8-15(9-11-30)31-23-21(29(2)24(31)33)13-25-20-7-6-18(27-22(20)23)16-4-3-5-19-17(16)12-26-28-19/h3-7,12-13,15H,8-11H2,1-2H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50331911
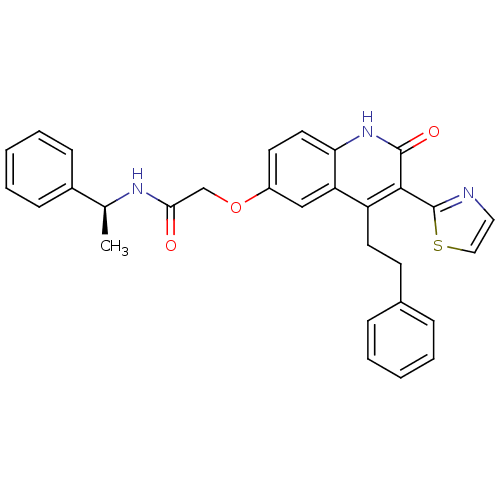
((S)-2-(2-oxo-4-phenethyl-3-(thiazol-2-yl)-1,2-dihy...)Show SMILES C[C@H](NC(=O)COc1ccc2[nH]c(=O)c(-c3nccs3)c(CCc3ccccc3)c2c1)c1ccccc1 |r| Show InChI InChI=1S/C30H27N3O3S/c1-20(22-10-6-3-7-11-22)32-27(34)19-36-23-13-15-26-25(18-23)24(14-12-21-8-4-2-5-9-21)28(29(35)33-26)30-31-16-17-37-30/h2-11,13,15-18,20H,12,14,19H2,1H3,(H,32,34)(H,33,35)/t20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosien A2B receptor in human HMC-1 cells assessed as inhibition of NECA-induced IL-8 release after 6 hr by ELISA |
Bioorg Med Chem Lett 20: 7414-20 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.030
BindingDB Entry DOI: 10.7270/Q2WH2Q60 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219035

(CHEMBL243355 | N-{5-[1-(3-{[bis(4-fluorophenyl)ace...)Show SMILES CC(C)C(=O)Nc1cc(C2CCN(CCCNC(=O)C(c3ccc(F)cc3)c3ccc(F)cc3)CC2)c(F)cc1F Show InChI InChI=1S/C32H35F4N3O2/c1-20(2)31(40)38-29-18-26(27(35)19-28(29)36)21-12-16-39(17-13-21)15-3-14-37-32(41)30(22-4-8-24(33)9-5-22)23-6-10-25(34)11-7-23/h4-11,18-21,30H,3,12-17H2,1-2H3,(H,37,41)(H,38,40) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219047
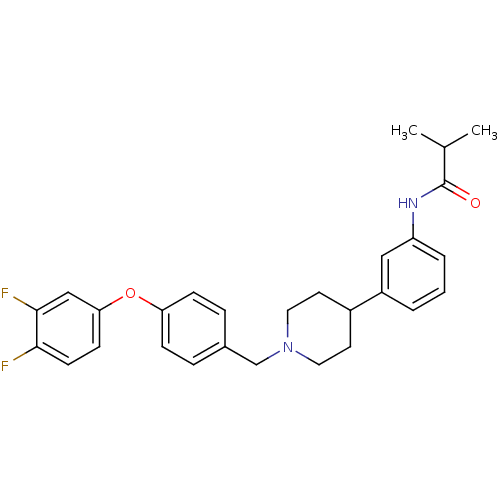
(CHEMBL245231 | N-(3-(1-(4-(3,4-difluorophenoxy)ben...)Show SMILES CC(C)C(=O)Nc1cccc(c1)C1CCN(Cc2ccc(Oc3ccc(F)c(F)c3)cc2)CC1 Show InChI InChI=1S/C28H30F2N2O2/c1-19(2)28(33)31-23-5-3-4-22(16-23)21-12-14-32(15-13-21)18-20-6-8-24(9-7-20)34-25-10-11-26(29)27(30)17-25/h3-11,16-17,19,21H,12-15,18H2,1-2H3,(H,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]T226296 from rat recombinant MCH1 receptor |
J Med Chem 50: 3883-90 (2007)
Article DOI: 10.1021/jm060383x
BindingDB Entry DOI: 10.7270/Q25D8RJM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219029

(CHEMBL390679 | N-{3-[1-(2-diphenylacetylamino-ethy...)Show SMILES CC(C)C(=O)Nc1cccc(c1)C1CCN(CCNC(=O)C(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C31H37N3O2/c1-23(2)30(35)33-28-15-9-14-27(22-28)24-16-19-34(20-17-24)21-18-32-31(36)29(25-10-5-3-6-11-25)26-12-7-4-8-13-26/h3-15,22-24,29H,16-21H2,1-2H3,(H,32,36)(H,33,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219023

(CHEMBL243138 | N-{3-[1-(3-diphenylacetylamino-prop...)Show SMILES CC(C)C(=O)Nc1cccc(c1)C1CCN(CCCNC(=O)C(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C32H39N3O2/c1-24(2)31(36)34-29-16-9-15-28(23-29)25-17-21-35(22-18-25)20-10-19-33-32(37)30(26-11-5-3-6-12-26)27-13-7-4-8-14-27/h3-9,11-16,23-25,30H,10,17-22H2,1-2H3,(H,33,37)(H,34,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50219037
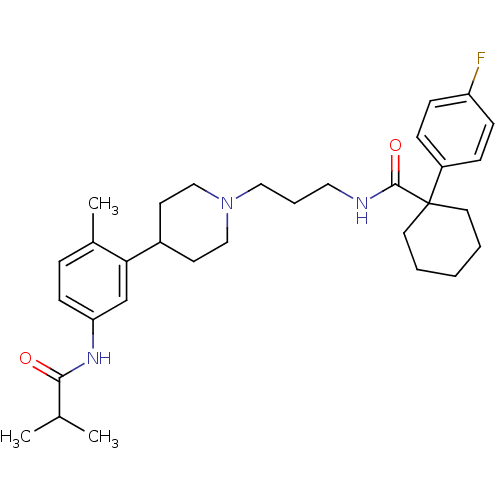
(1-(4-fluorophenyl)-N-(3-{4-[5-(isobutyrylamino)-2-...)Show SMILES CC(C)C(=O)Nc1ccc(C)c(c1)C1CCN(CCCNC(=O)C2(CCCCC2)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C32H44FN3O2/c1-23(2)30(37)35-28-13-8-24(3)29(22-28)25-14-20-36(21-15-25)19-7-18-34-31(38)32(16-5-4-6-17-32)26-9-11-27(33)12-10-26/h8-13,22-23,25H,4-7,14-21H2,1-3H3,(H,34,38)(H,35,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]SNAP 7941 from rat MCHR1 expressed in HEK293 cells |
J Med Chem 50: 3870-82 (2007)
Article DOI: 10.1021/jm060381c
BindingDB Entry DOI: 10.7270/Q2930SWM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428114

(CHEMBL2331662 | US8791131, 173)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O Show InChI InChI=1S/C23H24N6O2/c1-14-4-5-16(12-24-14)18-6-7-19-21(26-18)22-20(13-25-19)27(3)23(31)29(22)17-8-10-28(11-9-17)15(2)30/h4-7,12-13,17H,8-11H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data