

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301329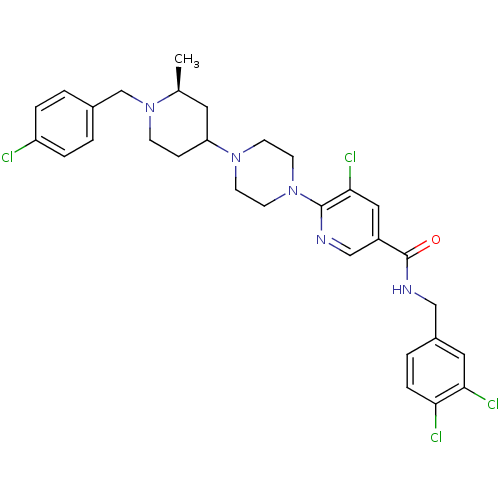 (5-chloro-6-(4-((2S)-1-(4-chlorobenzyl)-2-methylpip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301330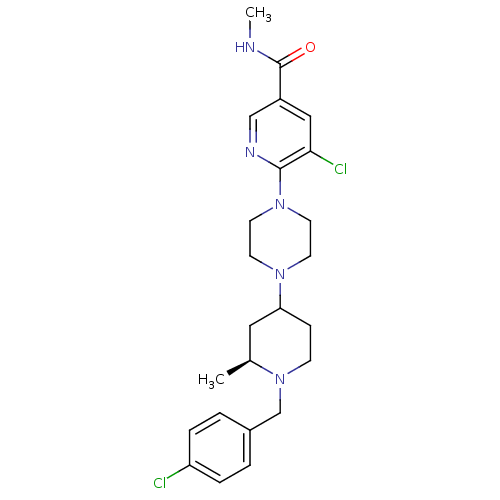 (5-chloro-6-(4-((2S)-1-(4-chlorobenzyl)-2-methylpip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301351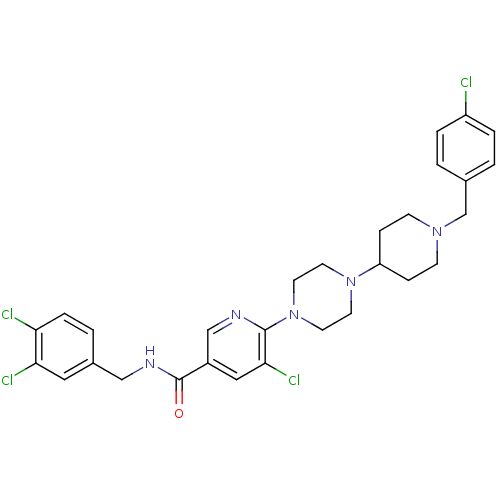 (5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-yl)pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50071558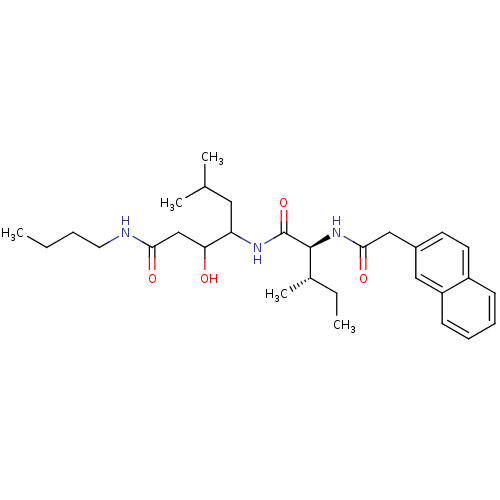 (3-Hydroxy-6-methyl-4-[(2S,3S)-3-methyl-2-(2-naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against plasmepsin-2 from Plasmodium falciparum | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50071554 (3-Hydroxy-4-[(2S,3S)-3-methyl-2-(2-naphthalen-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against cathepsin D | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301340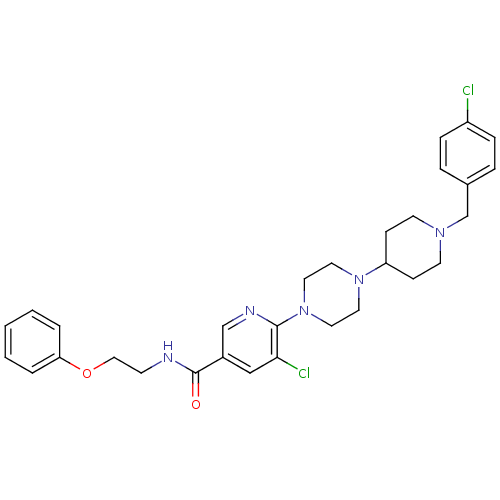 (5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-yl)pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301341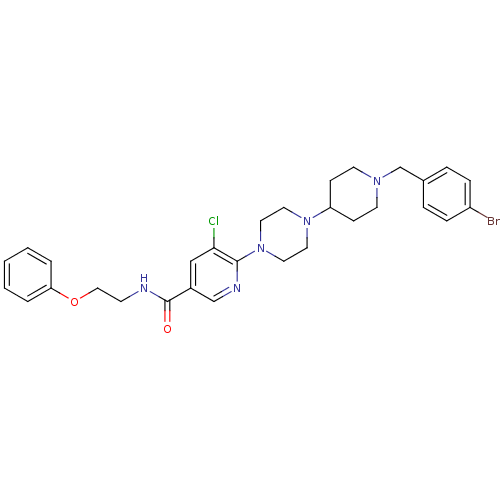 (6-(4-(1-(4-bromobenzyl)piperidin-4-yl)piperazin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301328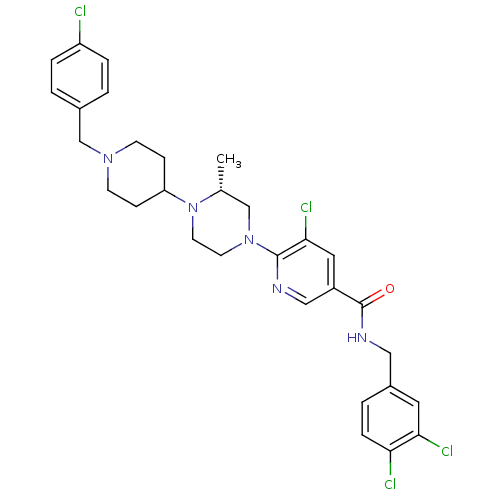 ((R)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50071554 (3-Hydroxy-4-[(2S,3S)-3-methyl-2-(2-naphthalen-2-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301326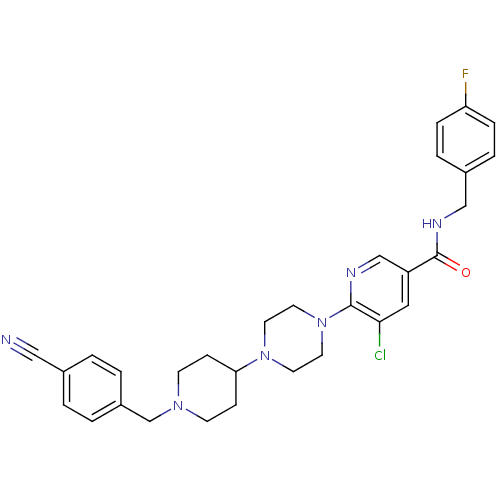 (5-chloro-6-(4-(1-(4-cyanobenzyl)piperidin-4-yl)pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301335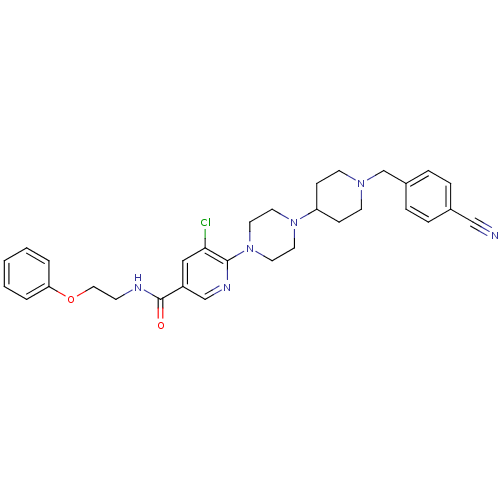 (5-chloro-6-(4-(1-(4-cyanobenzyl)piperidin-4-yl)pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50071561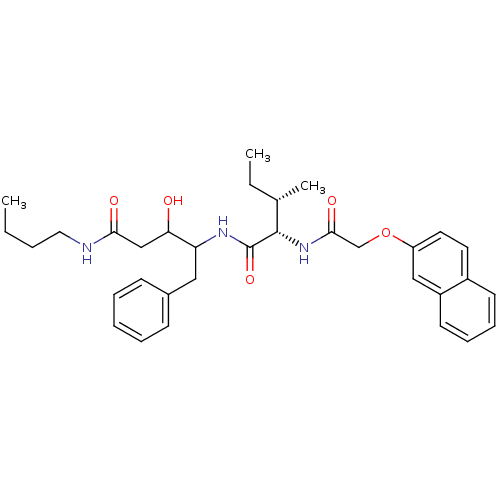 (3-Hydroxy-4-{(2S,3S)-3-methyl-2-[2-(naphthalen-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50071546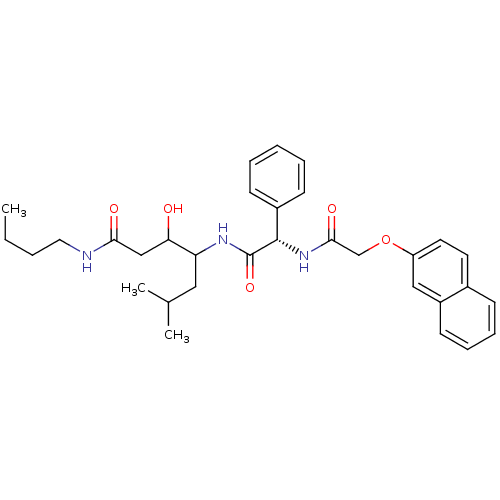 (3-Hydroxy-6-methyl-4-{(S)-2-[2-(naphthalen-2-yloxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against cathepsin D | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301344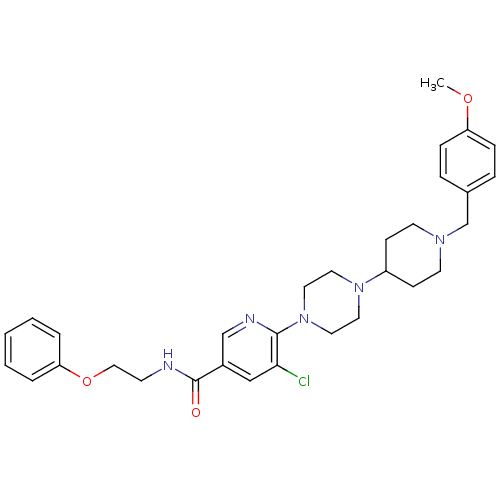 (5-chloro-6-(4-(1-(4-methoxybenzyl)piperidin-4-yl)p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301348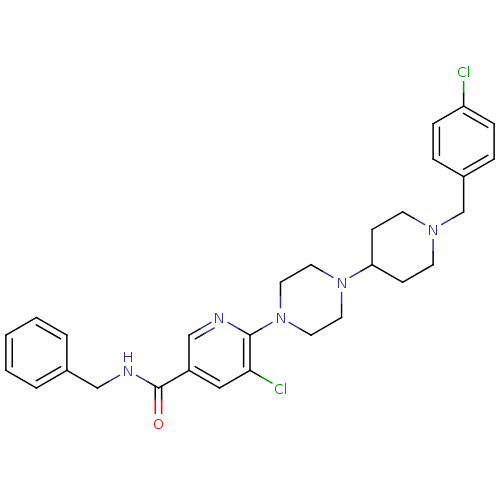 (CHEMBL577409 | N-benzyl-5-chloro-6-(4-(1-(4-chloro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301342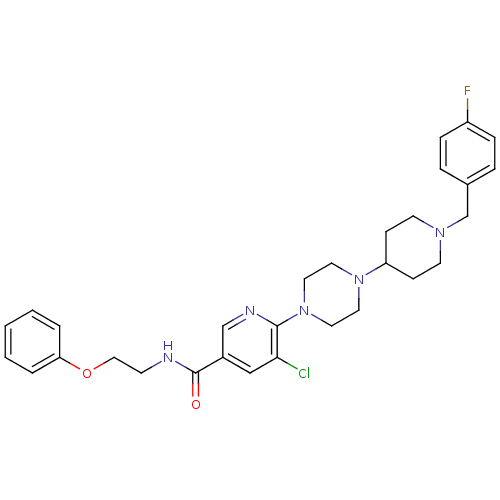 (5-chloro-6-(4-(1-(4-fluorobenzyl)piperidin-4-yl)pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50071555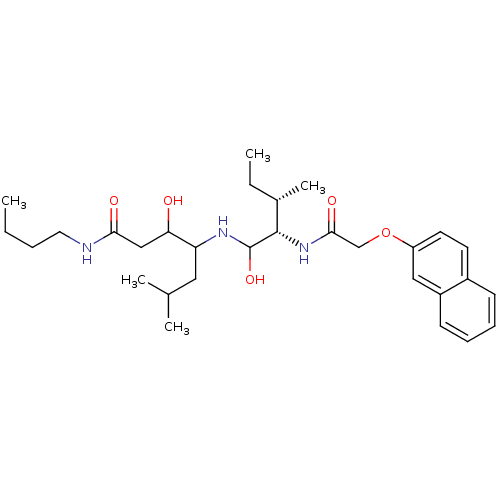 (3-Hydroxy-4-{(2S,3S)-1-hydroxy-3-methyl-2-[2-(naph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50071562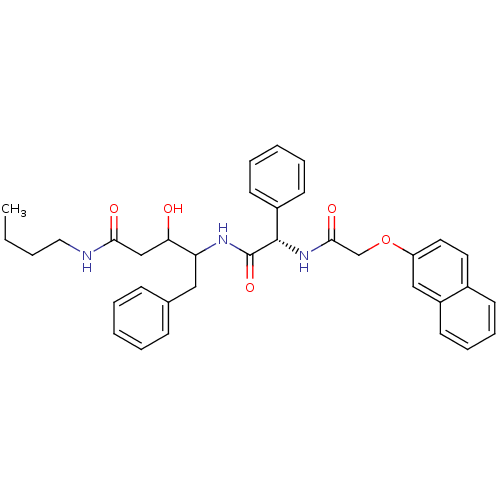 (3-Hydroxy-4-{(S)-2-[2-(naphthalen-2-yloxy)-acetyla...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against cathepsin D | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50071559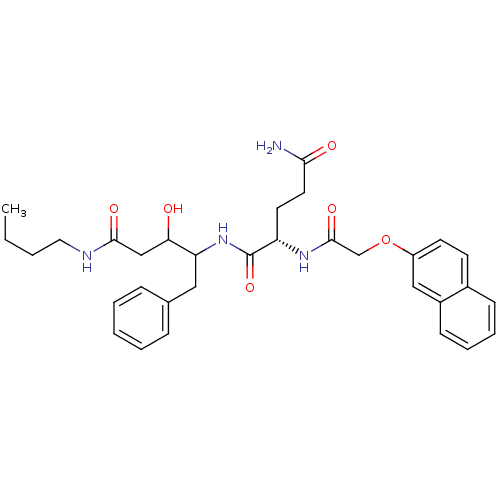 ((S)-2-[2-(Naphthalen-2-yloxy)-acetylamino]-pentane...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against cathepsin D | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50071550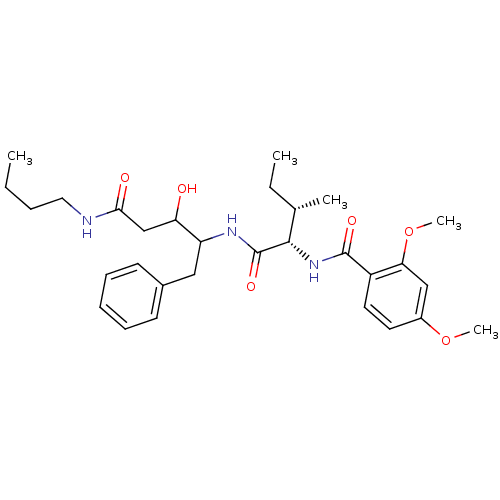 (CHEMBL74562 | N-[(1S,2S)-1-(1-Benzyl-3-butylcarbam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50071556 (CHEMBL74943 | N-[(1S,2S)-1-(1-Benzyl-3-butylcarbam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301339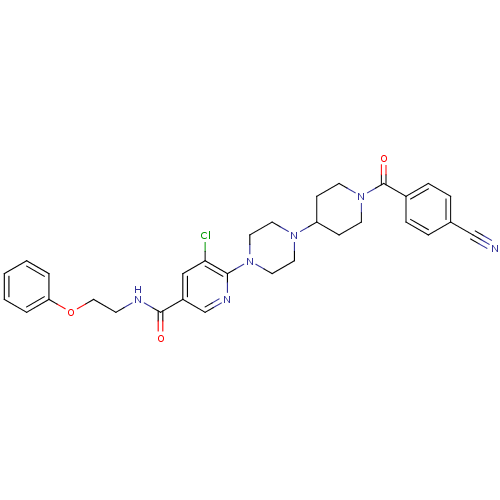 (5-chloro-6-(4-(1-(4-cyanobenzoyl)piperidin-4-yl)pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50071558 (3-Hydroxy-6-methyl-4-[(2S,3S)-3-methyl-2-(2-naphth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against cathepsin D | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301325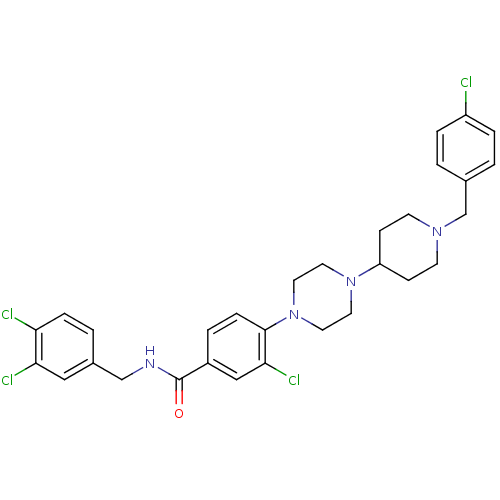 (3-chloro-4-(4-(1-(4-chlorobenzyl)piperidin-4-yl)pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301347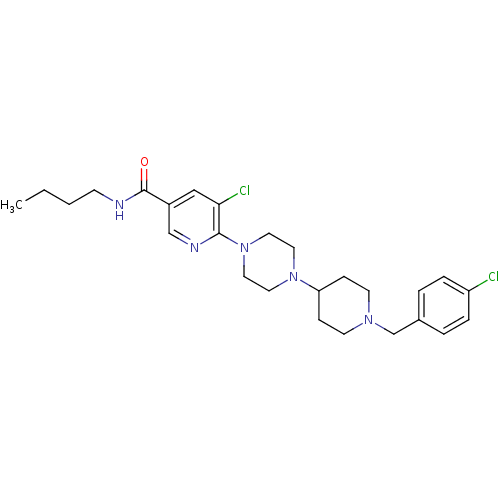 (CHEMBL579195 | N-butyl-5-chloro-6-(4-(1-(4-chlorob...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50071552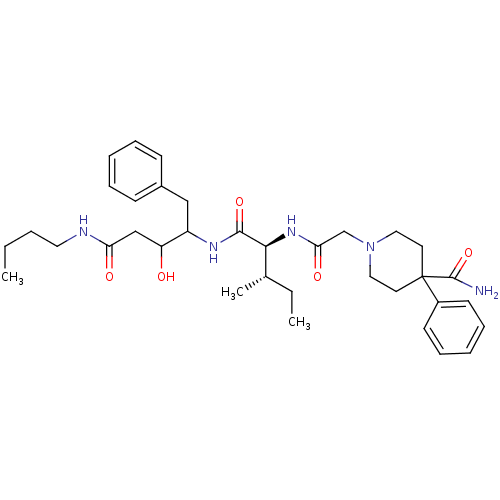 (1-{[(1S,2S)-1-(1-Benzyl-3-butylcarbamoyl-2-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301350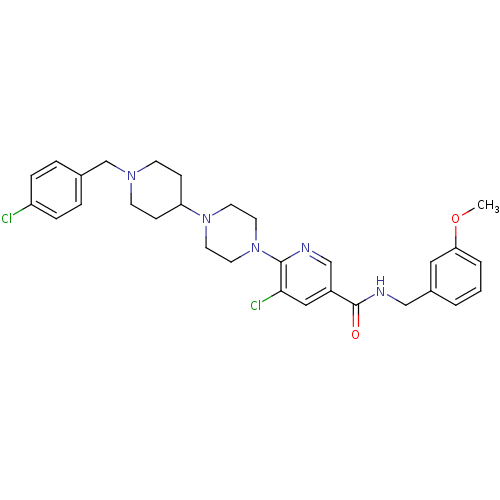 (5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-yl)pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50146770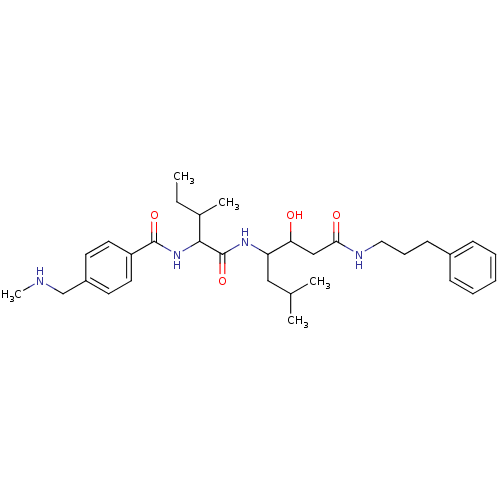 (CHEMBL94646 | N-(1-{1-[1-Hydroxy-2-(3-phenyl-propy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301349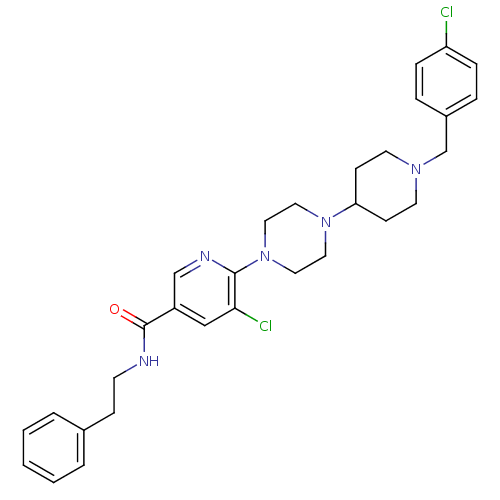 (5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-yl)pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50072543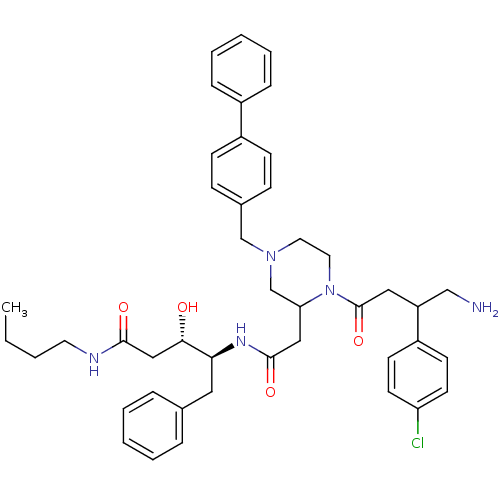 ((3S,4S)-4-(2-{1-[4-Amino-3-(4-chloro-phenyl)-butyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against plasmepsin II | Bioorg Med Chem Lett 8: 3203-6 (1999) BindingDB Entry DOI: 10.7270/Q2D21WSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50071551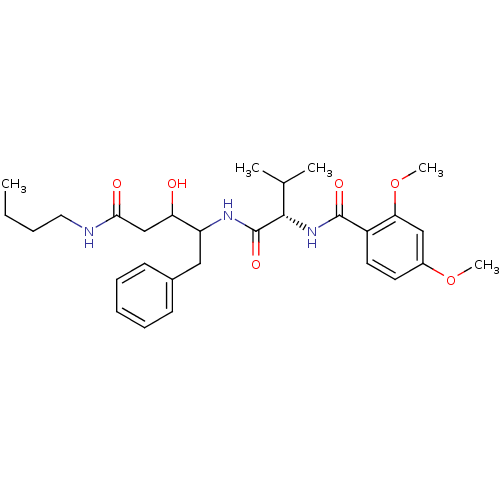 (CHEMBL427946 | N-[(S)-1-(1-Benzyl-3-butylcarbamoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301343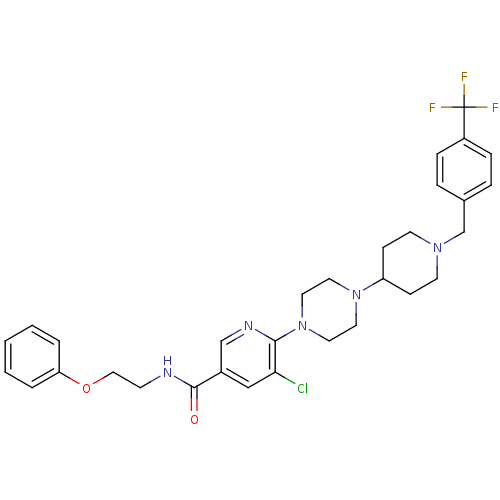 (5-chloro-N-(2-phenoxyethyl)-6-(4-(1-(4-(trifluorom...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50071562 (3-Hydroxy-4-{(S)-2-[2-(naphthalen-2-yloxy)-acetyla...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50071555 (3-Hydroxy-4-{(2S,3S)-1-hydroxy-3-methyl-2-[2-(naph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against cathepsin D | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301346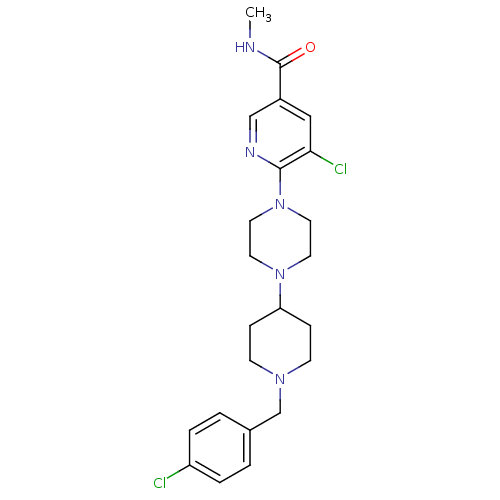 (5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-yl)pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50071549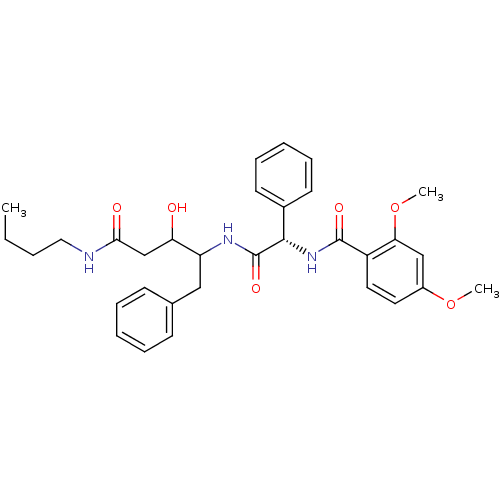 (CHEMBL309056 | N-[(S)-(1-Benzyl-3-butylcarbamoyl-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50071553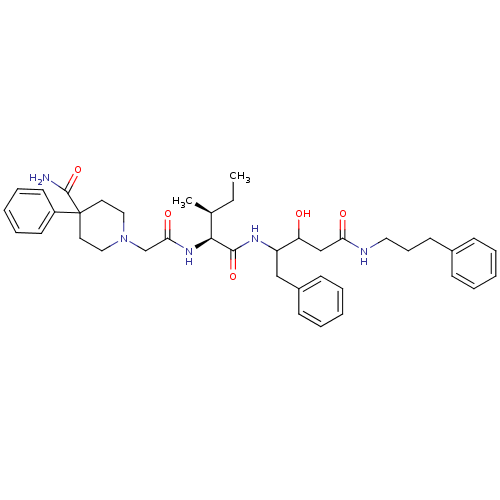 (1-({(1S,2S)-1-[1-Benzyl-2-hydroxy-3-(3-phenyl-prop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50071547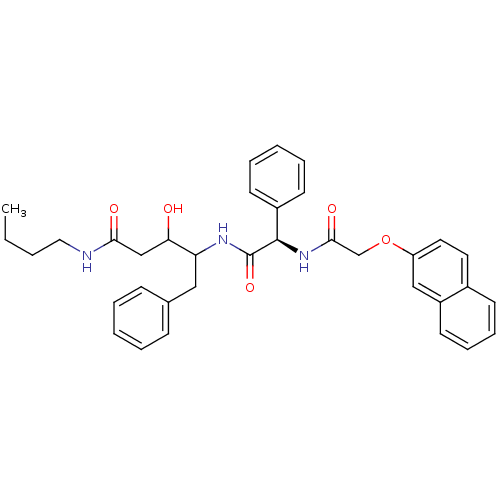 (3-Hydroxy-4-{(R)-2-[2-(naphthalen-2-yloxy)-acetyla...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against cathepsin D | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301354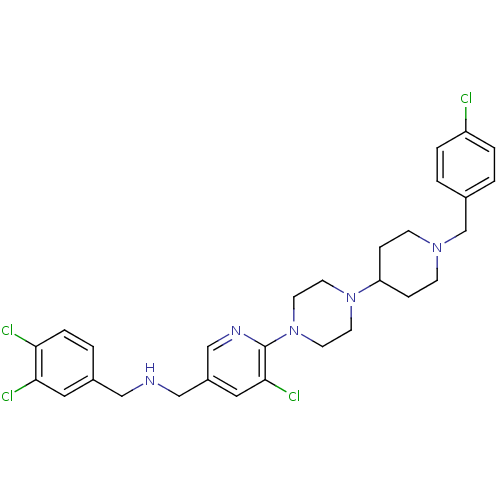 (1-(5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50071557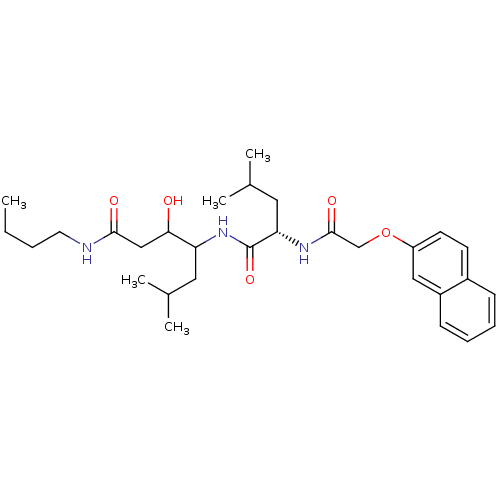 (3-Hydroxy-6-methyl-4-{(S)-4-methyl-2-[2-(naphthale...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against cathepsin D | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50072545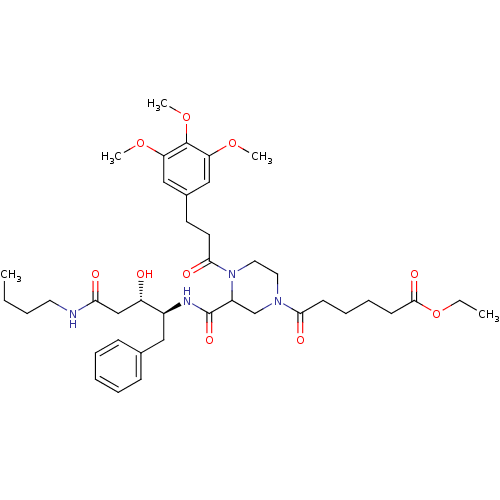 (6-{3-((1S,2S)-1-Benzyl-3-butylcarbamoyl-2-hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against cathepsin D | Bioorg Med Chem Lett 8: 3203-6 (1999) BindingDB Entry DOI: 10.7270/Q2D21WSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50146770 (CHEMBL94646 | N-(1-{1-[1-Hydroxy-2-(3-phenyl-propy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against cathepsin D | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50071560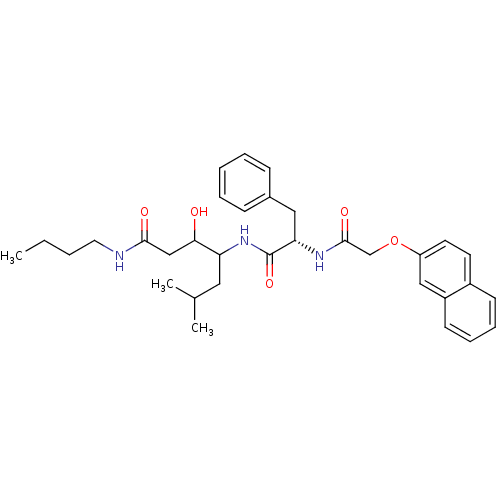 (3-Hydroxy-6-methyl-4-{(S)-2-[2-(naphthalen-2-yloxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against cathepsin D | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50071551 (CHEMBL427946 | N-[(S)-1-(1-Benzyl-3-butylcarbamoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against cathepsin D | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50072542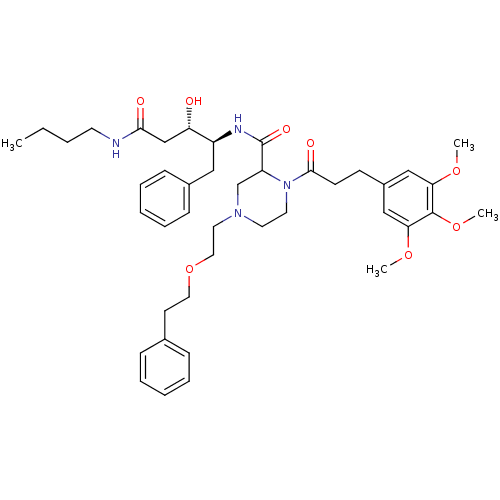 (4-(2-Phenethyloxy-ethyl)-1-[3-(3,4,5-trimethoxy-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against cathepsin D | Bioorg Med Chem Lett 8: 3203-6 (1999) BindingDB Entry DOI: 10.7270/Q2D21WSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301336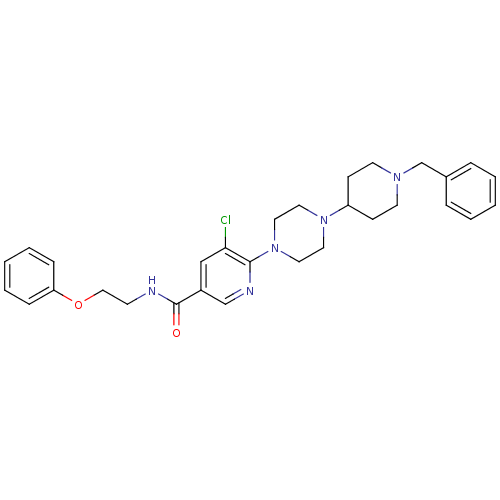 (6-(4-(1-benzylpiperidin-4-yl)piperazin-1-yl)-5-chl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301352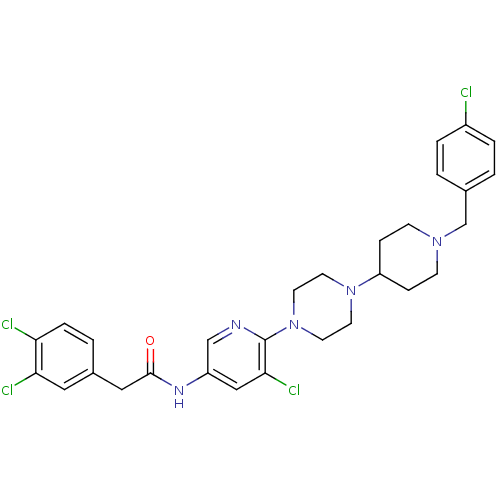 (CHEMBL584079 | N-(5-chloro-6-(4-(1-(4-chlorobenzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301334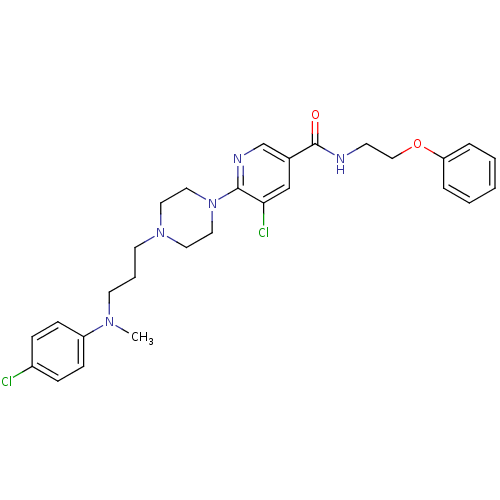 (5-chloro-6-(4-(3-((4-chlorophenyl)(methyl)amino)pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50071550 (CHEMBL74562 | N-[(1S,2S)-1-(1-Benzyl-3-butylcarbam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against cathepsin D | Bioorg Med Chem Lett 8: 2315-20 (1999) BindingDB Entry DOI: 10.7270/Q2SN09G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50301331 (5-chloro-6-(1'-(4-chlorobenzyl)-4,4'-bipiperidin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting | Bioorg Med Chem Lett 19: 5205-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.020 BindingDB Entry DOI: 10.7270/Q21V5F18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 224 total ) | Next | Last >> |