| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 2C |
|---|
| Ligand | BDBM232202 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | [3H]Mesulergine Binding Assay |
|---|
| pH | 7.4±n/a |
|---|
| IC50 | 8.8e+3±n/a nM |
|---|
| Comments | extracted |
|---|
| Citation |  Jung, JC; Min, D; Kim, H; Jang, S; Lee, Y; Park, W; Khan, IA; Moon, HI; Jung, M; Oh, S Design, synthesis, and biological evaluation of 3,4,5-trimethoxyphenyl acrylamides as antinarcotic agents. J Enzyme Inhib Med Chem25:38-43 (2010) [PubMed] Article Jung, JC; Min, D; Kim, H; Jang, S; Lee, Y; Park, W; Khan, IA; Moon, HI; Jung, M; Oh, S Design, synthesis, and biological evaluation of 3,4,5-trimethoxyphenyl acrylamides as antinarcotic agents. J Enzyme Inhib Med Chem25:38-43 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 2C |
|---|
| Name: | 5-hydroxytryptamine receptor 2C |
|---|
| Synonyms: | 5-HT-1C | 5-HT-2C | 5-HT1C | 5-HT2C | 5-HT2C-INI | 5-HT2c VGI | 5-HTR2C | 5-hydroxytryptamine receptor 1C | 5-hydroxytryptamine receptor 2C (5-HT-2C) | 5-hydroxytryptamine receptor 2C (5HT-2C) | 5HT-1C | 5HT2C_HUMAN | HTR1C | HTR2C | Serotonin (5-HT3) receptor | Serotonin 2c (5-HT2c) receptor | Serotonin Receptor 2C |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 51836.79 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P28335 |
|---|
| Residue: | 458 |
|---|
| Sequence: | MVNLRNAVHSFLVHLIGLLVWQSDISVSPVAAIVTDIFNTSDGGRFKFPDGVQNWPALSI
VIIIIMTIGGNILVIMAVSMEKKLHNATNYFLMSLAIADMLVGLLVMPLSLLAILYDYVW
PLPRYLCPVWISLDVLFSTASIMHLCAISLDRYVAIRNPIEHSRFNSRTKAIMKIAIVWA
ISIGVSVPIPVIGLRDEEKVFVNNTTCVLNDPNFVLIGSFVAFFIPLTIMVITYCLTIYV
LRRQALMLLHGHTEEPPGLSLDFLKCCKRNTAEEENSANPNQDQNARRRKKKERRPRGTM
QAINNERKASKVLGIVFFVFLIMWCPFFITNILSVLCEKSCNQKLMEKLLNVFVWIGYVC
SGINPLVYTLFNKIYRRAFSNYLRCNYKVEKKPPVRQIPRVAATALSGRELNVNIYRHTN
EPVIEKASDNEPGIEMQVENLELPVNPSSVVSERISSV
|
|
|
|---|
| BDBM232202 |
|---|
| n/a |
|---|
| Name | BDBM232202 |
|---|
| Synonyms: | (E)-N-Octyl-3-(3,4,5-trimethoxyphenyl) acrylamide (1e) |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H31NO4 |
|---|
| Mol. Mass. | 349.4644 |
|---|
| SMILES | CCCCCCCCNC(=O)\C=C\c1cc(OC)c(OC)c(OC)c1 |
|---|
| Structure | 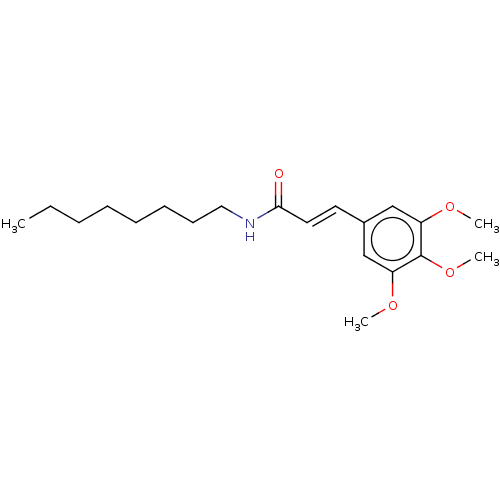
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Jung, JC; Min, D; Kim, H; Jang, S; Lee, Y; Park, W; Khan, IA; Moon, HI; Jung, M; Oh, S Design, synthesis, and biological evaluation of 3,4,5-trimethoxyphenyl acrylamides as antinarcotic agents. J Enzyme Inhib Med Chem25:38-43 (2010) [PubMed] Article
Jung, JC; Min, D; Kim, H; Jang, S; Lee, Y; Park, W; Khan, IA; Moon, HI; Jung, M; Oh, S Design, synthesis, and biological evaluation of 3,4,5-trimethoxyphenyl acrylamides as antinarcotic agents. J Enzyme Inhib Med Chem25:38-43 (2010) [PubMed] Article