

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50527134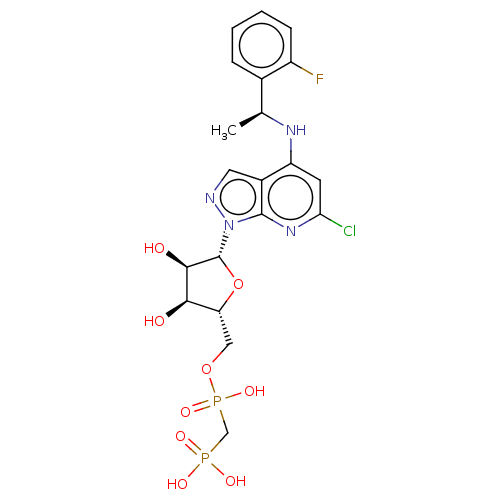 (CHEMBL4471306 | US20230295213, Compound a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human CD73 | J Med Chem 63: 2941-2957 (2020) Article DOI: 10.1021/acs.jmedchem.9b01611 BindingDB Entry DOI: 10.7270/Q2NS0ZBH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50049553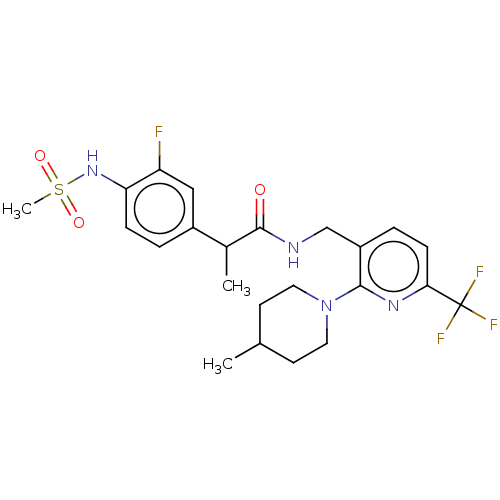 (CHEMBL2177428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activity by FLIPR assay | Eur J Med Chem 93: 101-8 (2015) Article DOI: 10.1016/j.ejmech.2015.02.001 BindingDB Entry DOI: 10.7270/Q2N0188S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50073160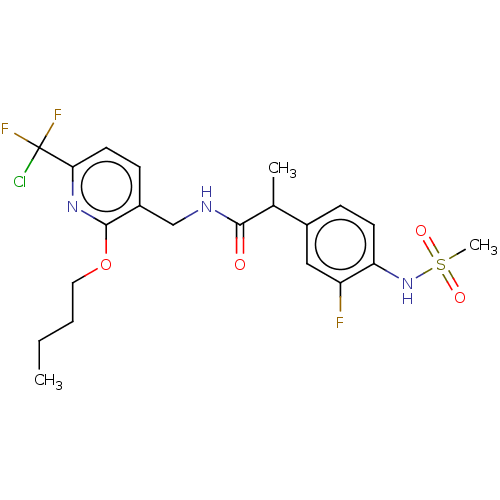 (CHEMBL3407762) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activity by FLIPR assay | Eur J Med Chem 93: 101-8 (2015) Article DOI: 10.1016/j.ejmech.2015.02.001 BindingDB Entry DOI: 10.7270/Q2N0188S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM86549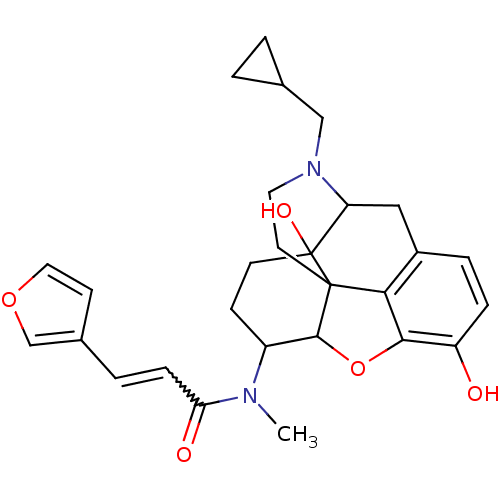 (CAS_213055 | NSC_213055 | TRK-820) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by PDSP Ki Database | J Pharmacol Exp Ther 312: 220-30 (2005) Article DOI: 10.1124/jpet.104.073668 BindingDB Entry DOI: 10.7270/Q26T0K6G | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50073159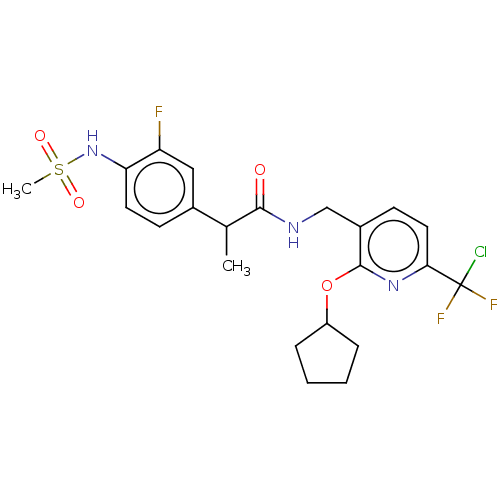 (CHEMBL3407765) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activity by FLIPR assay | Eur J Med Chem 93: 101-8 (2015) Article DOI: 10.1016/j.ejmech.2015.02.001 BindingDB Entry DOI: 10.7270/Q2N0188S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50366620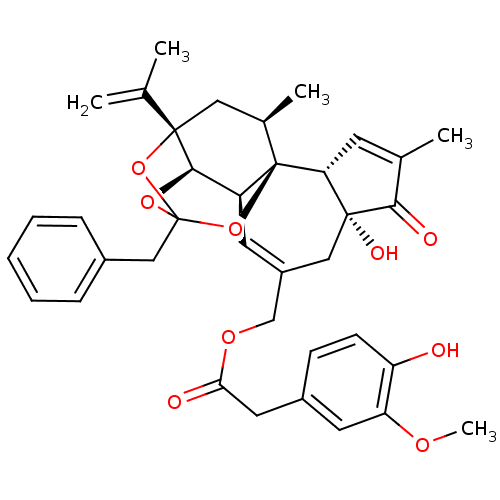 (RESINIFERATOXIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0426 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166333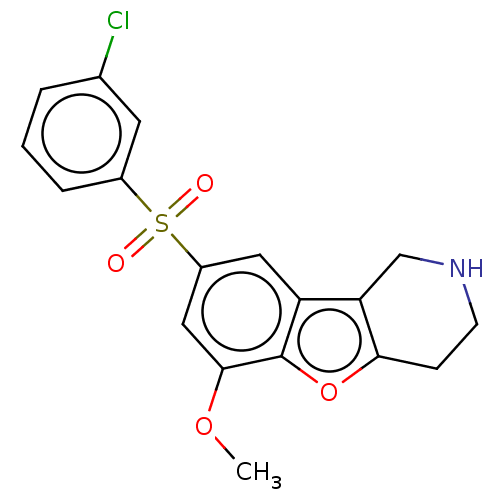 (US9067949, 190) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50071654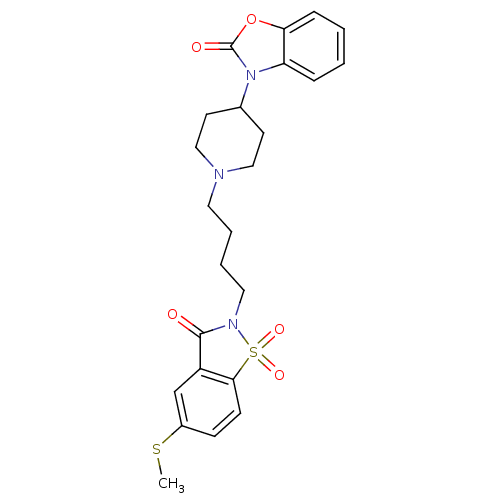 (3-{1-[4-(5-Methylsulfanyl-1,1,3-trioxo-1,3-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Ability to displace beta ([125I]-iodo-4-hydroxyphenyl)-ethylaminomethyl tetralone from human cloned Alpha-1a adrenergic receptor stably expressed in ... | Bioorg Med Chem Lett 8: 2467-72 (1999) BindingDB Entry DOI: 10.7270/Q2NP23K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50071655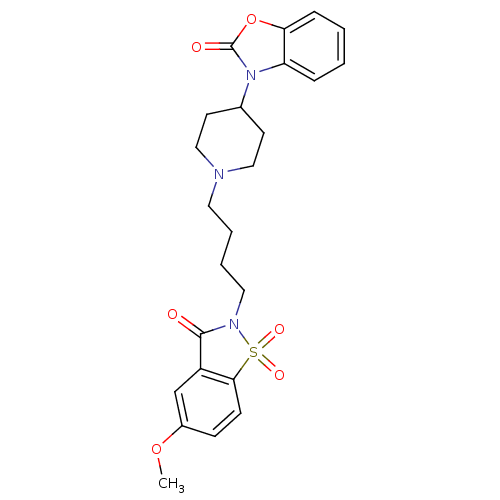 (3-{1-[4-(5-Methoxy-1,1,3-trioxo-1,3-dihydro-1lambd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Ability to displace beta ([125I]-iodo-4-hydroxyphenyl)-ethylaminomethyl tetralone from human cloned Alpha-1a adrenergic receptor stably expressed in ... | Bioorg Med Chem Lett 8: 2467-72 (1999) BindingDB Entry DOI: 10.7270/Q2NP23K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50558721 (CHEMBL4784791) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZG6WWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50256671 (CHEMBL4085431) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Design and Synthesis Section, Molecular Targets and Medications Discovery Branch, Intramural Research Program, National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Al Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain after 60 mins by liquid scintillation counting method | Bioorg Med Chem 25: 2406-2422 (2017) Article DOI: 10.1016/j.bmc.2017.02.064 BindingDB Entry DOI: 10.7270/Q2765HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50247744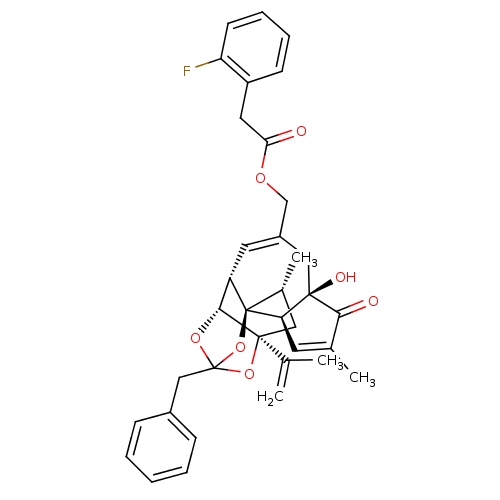 (CHEMBL504725 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM86549 (CAS_213055 | NSC_213055 | TRK-820) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by PDSP Ki Database | J Pharmacol Exp Ther 312: 220-30 (2005) Article DOI: 10.1124/jpet.104.073668 BindingDB Entry DOI: 10.7270/Q26T0K6G | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50071647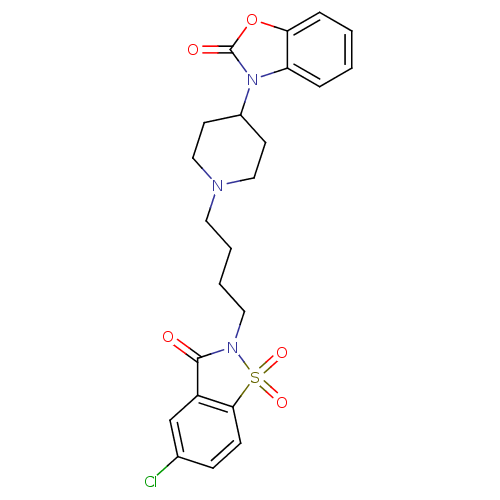 (3-{1-[4-(5-Chloro-1,1,3-trioxo-1,3-dihydro-1lambda...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Compound was evaluated for its affinity for Alpha-1a adrenergic receptor in human aorta preparations | Bioorg Med Chem Lett 8: 2467-72 (1999) BindingDB Entry DOI: 10.7270/Q2NP23K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166213 (US9067949, 70) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0830 | -57.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50071652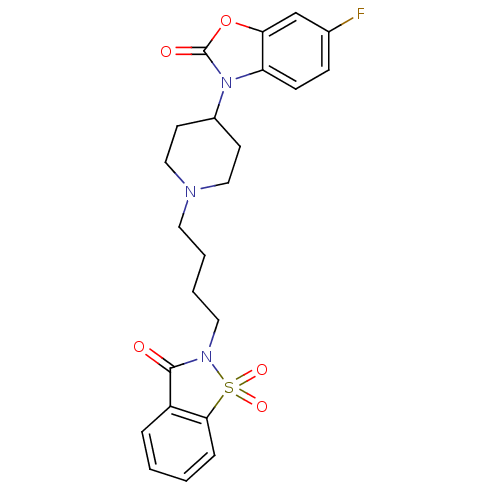 (6-Fluoro-3-{1-[4-(1,1,3-trioxo-1,3-dihydro-1lambda...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Ability to displace beta ([125I]-iodo-4-hydroxyphenyl)-ethylamino methyl tetralone from human cloned Alpha-1a adrenergic receptor stably expressed in... | Bioorg Med Chem Lett 8: 2467-72 (1999) BindingDB Entry DOI: 10.7270/Q2NP23K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM214798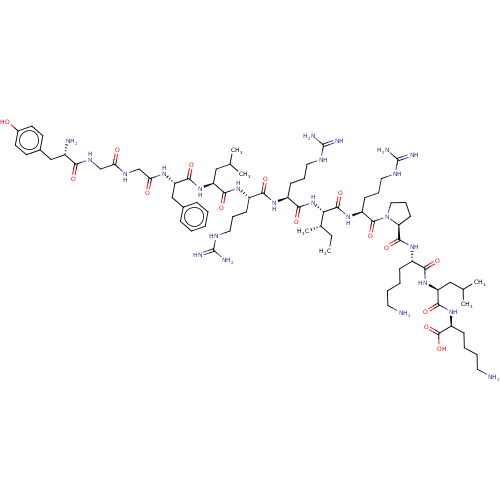 (Dynorphin A (1-17) | YGGFLRRIRPKLK) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZG6WWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50491858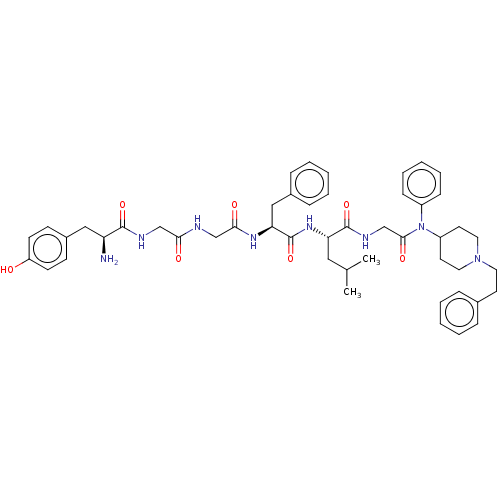 (CHEMBL2387338) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cell membranes | Bioorg Med Chem Lett 23: 3434-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.065 BindingDB Entry DOI: 10.7270/Q2T72MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50071651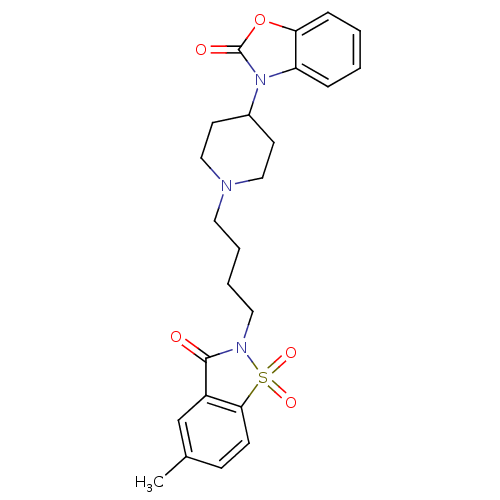 (3-{1-[4-(5-Methyl-1,1,3-trioxo-1,3-dihydro-1lambda...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Ability to displace beta ([125I]-iodo-4-hydroxyphenyl)-ethylaminomethyl tetralone from human cloned Alpha-1a adrenergic receptor stably expressed in ... | Bioorg Med Chem Lett 8: 2467-72 (1999) BindingDB Entry DOI: 10.7270/Q2NP23K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50010704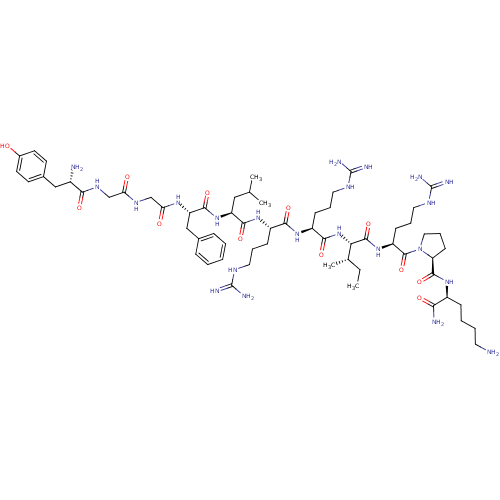 (CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZG6WWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166328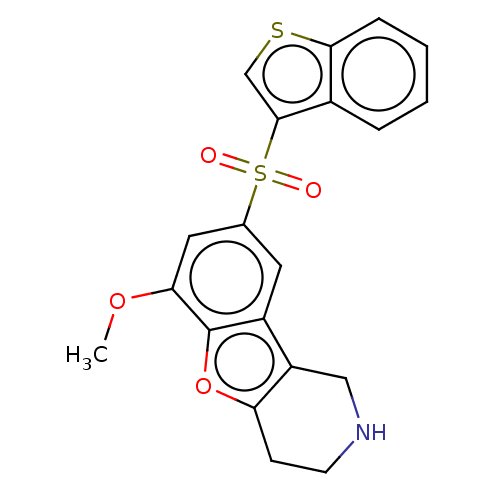 (US9067949, 185) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -57.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166358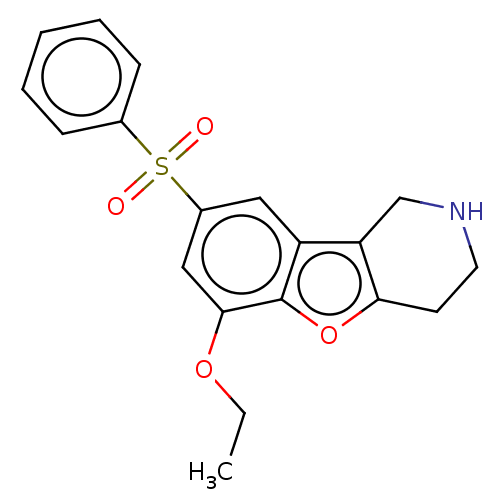 (US9067949, 215) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.110 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166331 (US9067949, 188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.110 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166303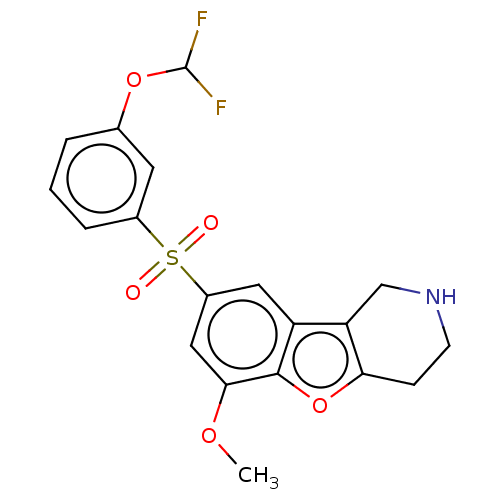 (US9067949, 160) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.110 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50071646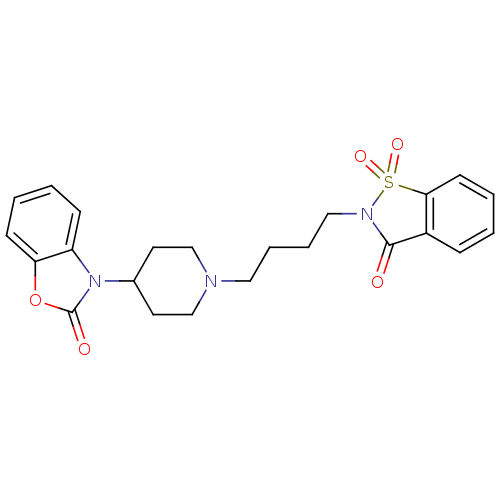 (3-{1-[4-(1,1,3-Trioxo-1,3-dihydro-1lambda*6*-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Ability to displace beta ([125I]-iodo-4-hydroxyphenyl)-ethylamino methyl tetralone from human cloned Alpha-1a adrenergic receptor stably expressed in... | Bioorg Med Chem Lett 8: 2467-72 (1999) BindingDB Entry DOI: 10.7270/Q2NP23K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50001157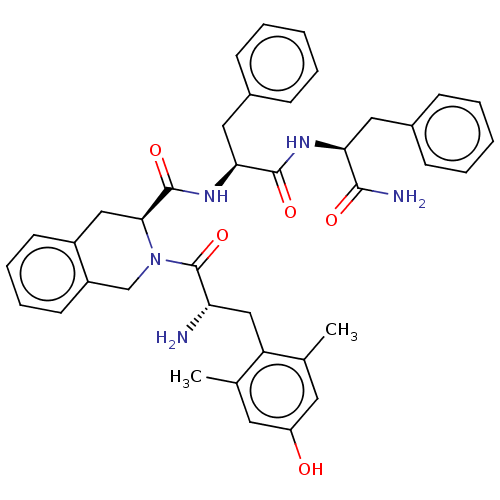 (CHEMBL538700) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in human/mouse HN9.10 cell membranes after 3 hrs by liquid scintillation countin... | ACS Med Chem Lett 4: 656-659 (2013) Article DOI: 10.1021/ml400115n BindingDB Entry DOI: 10.7270/Q2CN76VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50040123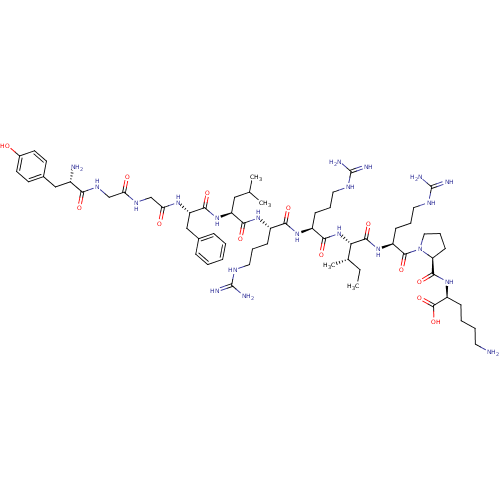 (CHEMBL438223 | HTry-Gly-Gly-Phe-Leu-Arg-Arg-lle-Ar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZG6WWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166197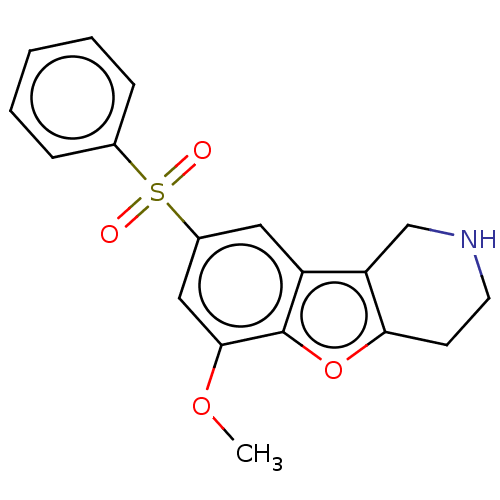 (US9067949, 54) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.120 | -56.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166244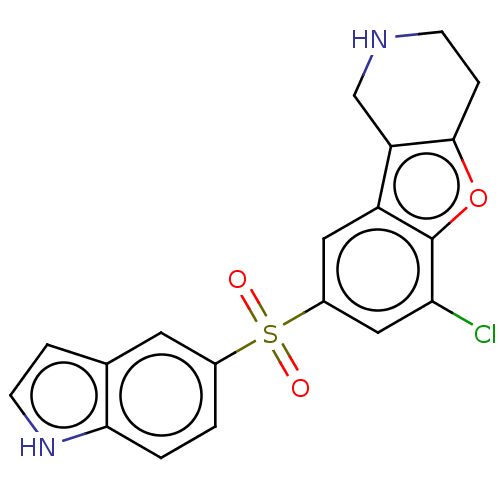 (US9067949, 101 | US9067949, 143) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.130 | -56.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166244 (US9067949, 101 | US9067949, 143) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.130 | -56.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166310 (US9067949, 167) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.130 | -56.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50586821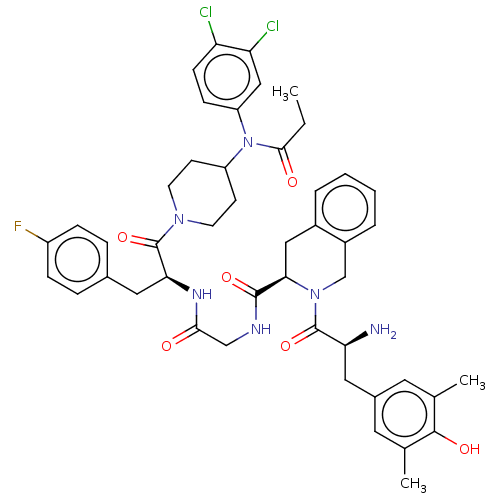 (CHEMBL5089012) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in mouse HN9.10 cells assessed as inhibition constant by radioligand binding a... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116509 BindingDB Entry DOI: 10.7270/Q2FX7FCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50321606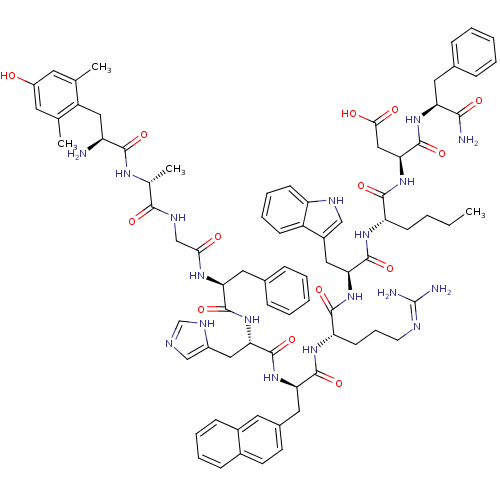 ((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells | Bioorg Med Chem Lett 20: 4080-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.078 BindingDB Entry DOI: 10.7270/Q2D79CCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50071661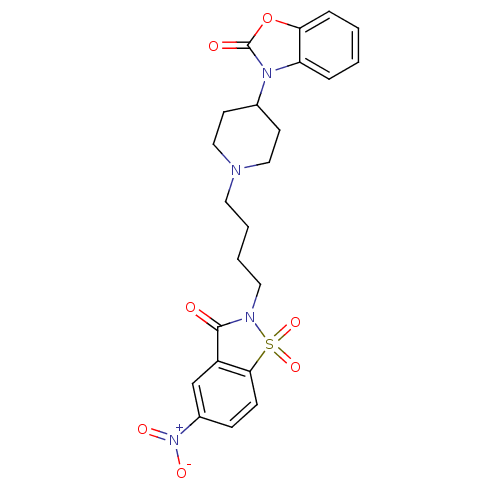 (3-{1-[4-(5-Nitro-1,1,3-trioxo-1,3-dihydro-1lambda*...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Ability to displace beta ([125I]-iodo-4-hydroxyphenyl)-ethylamino methyl tetralone from human cloned Alpha-1a adrenergic receptor stably expressed in... | Bioorg Med Chem Lett 8: 2467-72 (1999) BindingDB Entry DOI: 10.7270/Q2NP23K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50321606 ((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells | Bioorg Med Chem Lett 20: 4080-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.078 BindingDB Entry DOI: 10.7270/Q2D79CCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123599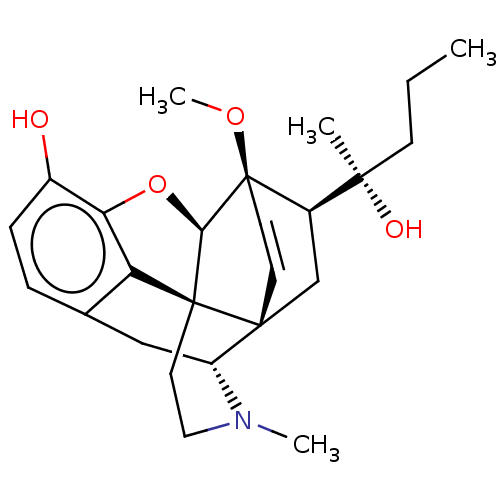 (ETORPHINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 25: 4689-92 (2015) Article DOI: 10.1016/j.bmcl.2015.06.092 BindingDB Entry DOI: 10.7270/Q2NV9M2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50367123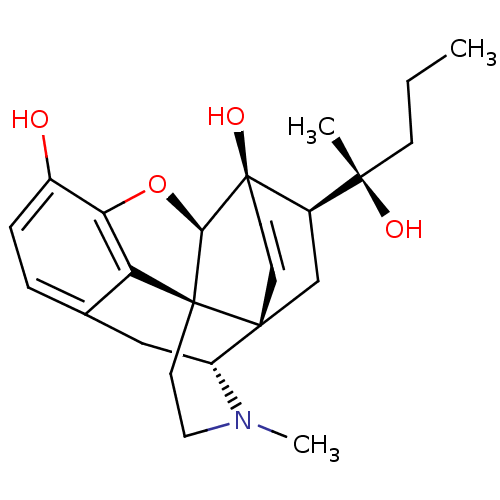 (ETORPHINE | M99) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 1301-4 (2009) Article DOI: 10.1016/j.bmcl.2009.01.078 BindingDB Entry DOI: 10.7270/Q2MP546B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50491860 (CHEMBL2387215) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cell membranes | Bioorg Med Chem Lett 23: 3434-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.065 BindingDB Entry DOI: 10.7270/Q2T72MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166225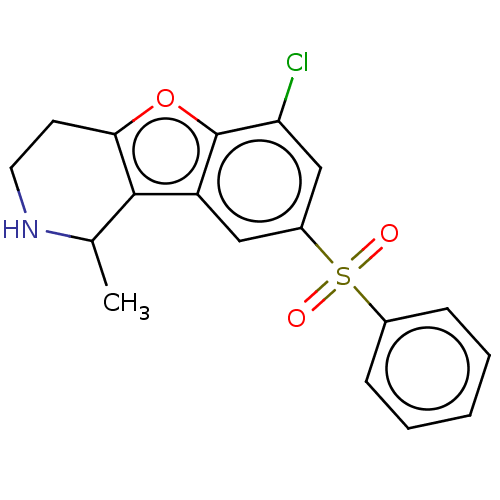 (US9067949, 81a | US9067949, 82a | US9067949, 82b) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.150 | -56.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166326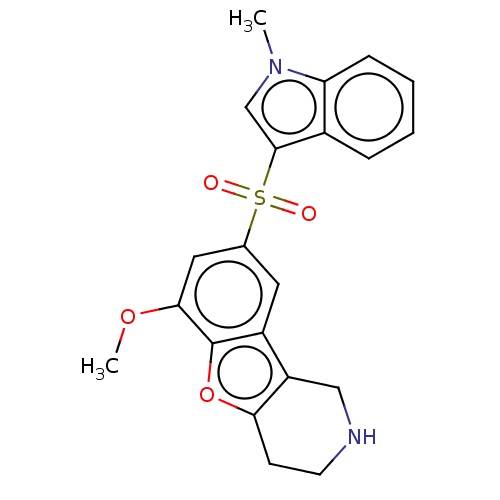 (US9067949, 183) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.160 | -55.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50071653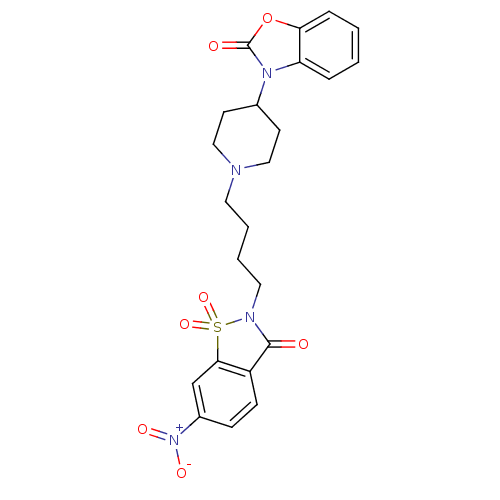 (3-{1-[4-(6-Nitro-1,1,3-trioxo-1,3-dihydro-1lambda*...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Ability to displace beta ([125I]-iodo-4-hydroxyphenyl)-ethylaminomethyl tetralone from human cloned Alpha-1a adrenergic receptor stably expressed in ... | Bioorg Med Chem Lett 8: 2467-72 (1999) BindingDB Entry DOI: 10.7270/Q2NP23K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166327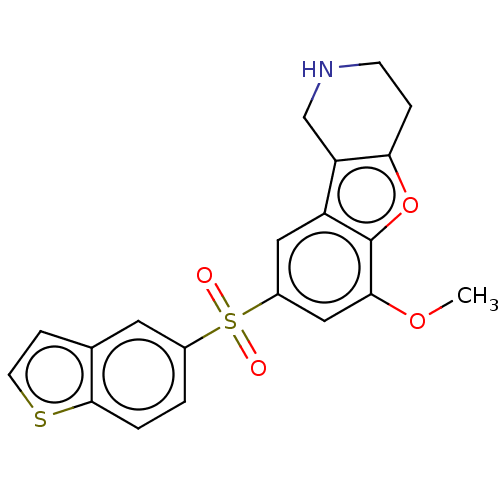 (US9067949, 184) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.160 | -55.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166193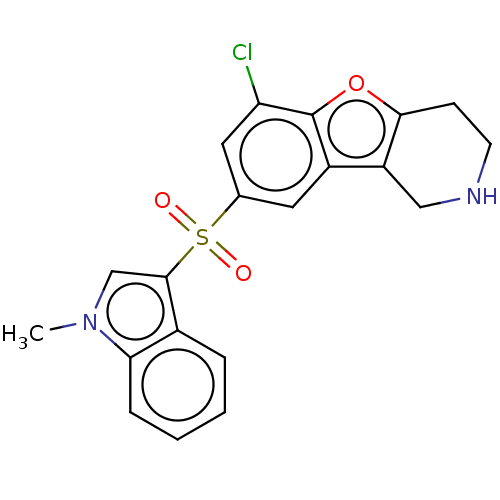 (US9067949, 50) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.170 | -55.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50491854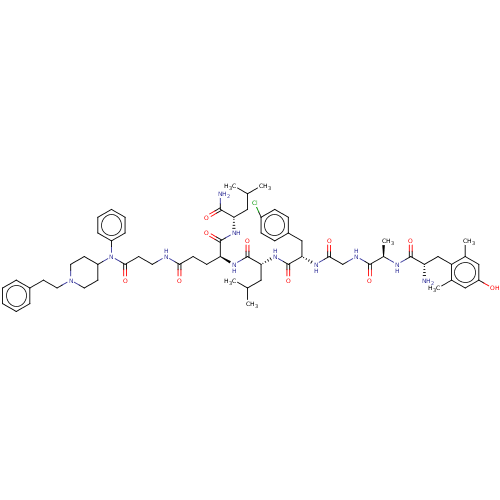 (CHEMBL2387327) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cell membranes | Bioorg Med Chem Lett 23: 3434-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.065 BindingDB Entry DOI: 10.7270/Q2T72MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50071647 (3-{1-[4-(5-Chloro-1,1,3-trioxo-1,3-dihydro-1lambda...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Compound was evaluated for its affinity for alpha 1a receptor in human prostate tissue preparations | Bioorg Med Chem Lett 8: 2467-72 (1999) BindingDB Entry DOI: 10.7270/Q2NP23K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166335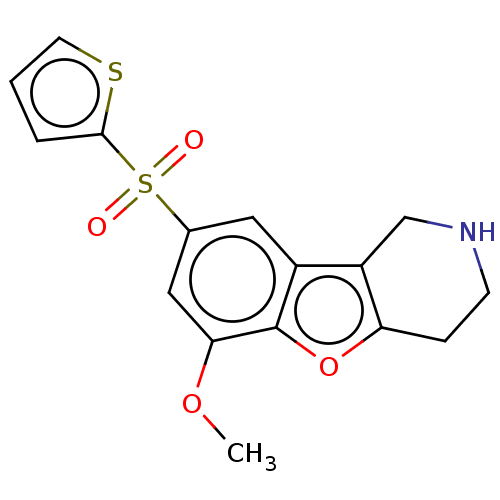 (US9067949, 192) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.170 | -55.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166306 (US9067949, 163) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.190 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50217956 ((1R,5S)-(+)-5-(3-hydroxyphenyl)-9-methylene-2-phen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human mu opioid receptor expressed in CHO cells | J Med Chem 50: 3765-76 (2007) Article DOI: 10.1021/jm061325e BindingDB Entry DOI: 10.7270/Q2D21XB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50217952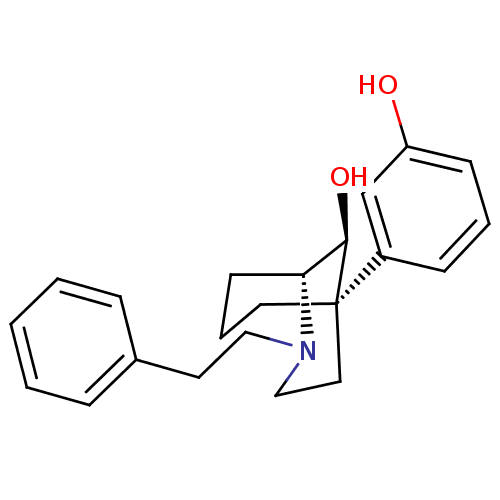 ((1R,5R,9S)-(-)-9-hydroxy-5-(3-hydroxyphenyl-2-phen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human mu opioid receptor expressed in CHO cells | J Med Chem 50: 3765-76 (2007) Article DOI: 10.1021/jm061325e BindingDB Entry DOI: 10.7270/Q2D21XB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166311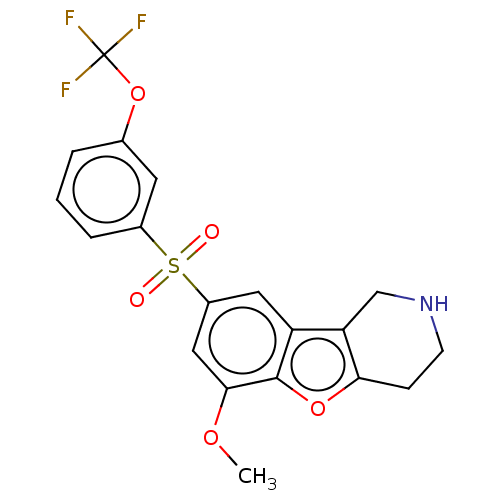 (US9067949, 168) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.190 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 14932 total ) | Next | Last >> |