| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetylcholinesterase |
|---|
| Ligand | BDBM240806 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | In Vitro AChE and BuChE Inhibition Assays |
|---|
| pH | 8±n/a |
|---|
| IC50 | 100.2±0.3 nM |
|---|
| Comments | extracted |
|---|
| Citation |  Li, Z; Wang, B; Hou, JQ; Huang, SL; Ou, TM; Tan, JH; An, LK; Li, D; Gu, LQ; Huang, ZS 2-(2-indolyl-)-4(3H)-quinazolines derivates as new inhibitors of AChE: design, synthesis, biological evaluation and molecular modelling. J Enzyme Inhib Med Chem28:583-92 (2013) [PubMed] Article Li, Z; Wang, B; Hou, JQ; Huang, SL; Ou, TM; Tan, JH; An, LK; Li, D; Gu, LQ; Huang, ZS 2-(2-indolyl-)-4(3H)-quinazolines derivates as new inhibitors of AChE: design, synthesis, biological evaluation and molecular modelling. J Enzyme Inhib Med Chem28:583-92 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetylcholinesterase |
|---|
| Name: | Acetylcholinesterase |
|---|
| Synonyms: | ACES_ELEEL | Acetylcholinesterase (AChE) | Acetylcholinesterase (EeAChE) | ache |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 71812.79 |
|---|
| Organism: | Electrophorus electricus (Electric eel) |
|---|
| Description: | n/a |
|---|
| Residue: | 633 |
|---|
| Sequence: | MKILDALLFPVIFIMFFIHLSIAQTDPELTIMTRLGQVQGTRLPVPDRSHVIAFLGIPFA
EPPLGKMRFKPPEPKKPWNDVFDARDYPSACYQYVDTSYPGFSGTEMWNPNRMMSEDCLY
LNVWVPATPRPHNLTVMVWIYGGGFYSGSSSLDVYDGRYLAHSEKVVVVSMNYRVSAFGF
LALNGSAEAPGNVGLLDQRLALQWVQDNIHFFGGNPKQVTIFGESAGAASVGMHLLSPDS
RPKFTRAILQSGVPNGPWRTVSFDEARRRAIKLGRLVGCPDGNDTDLIDCLRSKQPQDLI
DQEWLVLPFSGLFRFSFVPVIDGVVFPDTPEAMLNSGNFKDTQILLGVNQNEGSYFLIYG
APGFSKDNESLITREDFLQGVKMSVPHANEIGLEAVILQYTDWMDEDNPIKNREAMDDIV
GDHNVVCPLQHFAKMYAQYSILQGQTGTASQGNLGWGNSGSASNSGNSQVSVYLYMFDHR
ASNLVWPEWMGVIHGYEIEFVFGLPLEKRLNYTLEEEKLSRRMMKYWANFARTGNPNINV
DGSIDSRRRWPVFTSTEQKHVGLNTDSLKVHKGLKSQFCALWNRFLPRLLNVTENIDDAE
RQWKAEFHRWSSYMMHWKNQFDHYSKQERCTNL
|
|
|
|---|
| BDBM240806 |
|---|
| n/a |
|---|
| Name | BDBM240806 |
|---|
| Synonyms: | N-(2-(1H-indol-2-yl)-4-oxo-3,4-dihydroquinazolin-6-yl)-3-(pyrrolidin-1-yl)propanamide (3g) |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H23N5O2 |
|---|
| Mol. Mass. | 401.461 |
|---|
| SMILES | O=C(CCN1CCCC1)Nc1ccc2nc([nH]c(=O)c2c1)-c1cc2ccccc2[nH]1 |
|---|
| Structure | 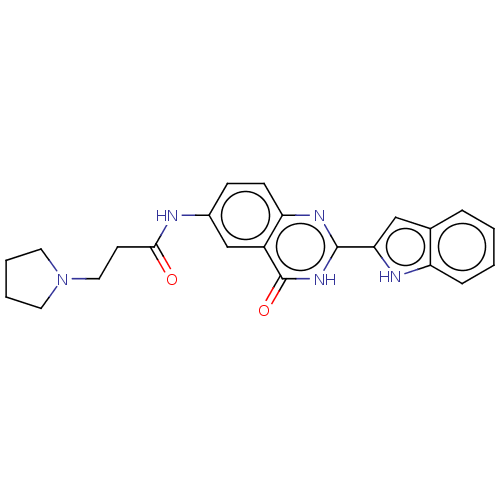
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Li, Z; Wang, B; Hou, JQ; Huang, SL; Ou, TM; Tan, JH; An, LK; Li, D; Gu, LQ; Huang, ZS 2-(2-indolyl-)-4(3H)-quinazolines derivates as new inhibitors of AChE: design, synthesis, biological evaluation and molecular modelling. J Enzyme Inhib Med Chem28:583-92 (2013) [PubMed] Article
Li, Z; Wang, B; Hou, JQ; Huang, SL; Ou, TM; Tan, JH; An, LK; Li, D; Gu, LQ; Huang, ZS 2-(2-indolyl-)-4(3H)-quinazolines derivates as new inhibitors of AChE: design, synthesis, biological evaluation and molecular modelling. J Enzyme Inhib Med Chem28:583-92 (2013) [PubMed] Article