

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | High affinity nerve growth factor receptor | ||
| Ligand | BDBM344324 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | TrkA ELISA Assay | ||
| IC50 | 23.4±n/a nM | ||
| Citation |  Allen, S; Andrews, SW; Condroski, KR; Haas, J; Huang, L; Jiang, Y; Kercher, T; Seo, J Substituted pyrazolo[1,5-a]pyrimidine compounds as Trk kinase inhibitors US Patent US10251889 Publication Date 4/9/2019 Allen, S; Andrews, SW; Condroski, KR; Haas, J; Huang, L; Jiang, Y; Kercher, T; Seo, J Substituted pyrazolo[1,5-a]pyrimidine compounds as Trk kinase inhibitors US Patent US10251889 Publication Date 4/9/2019 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| High affinity nerve growth factor receptor | |||
| Name: | High affinity nerve growth factor receptor | ||
| Synonyms: | 2.7.10.1 | MTC | NTRK1 | NTRK1/NTRK2 | NTRK1_HUMAN | Nerve growth factor receptor Trk-A | Neurotrophic tyrosine kinase receptor type 1 | Neurotrophic tyrosine kinase receptor type 1 (TrkA) | Synonyms=MTC | TRK | TRK1-transforming tyrosine kinase protein | TRKA | TRKA GN | TRKA GN | Trk-A | Tropomyosin alpha-3 chain/High affinity nerve growth factor receptor | Tropomyosin-related kinase A | Tropomyosin-related kinase A (TrkA) | Tyrosine kinase receptor | Tyrosine kinase receptor (Trk) | Tyrosine kinase receptor A | Tyrosine kinase receptor A (Trk A) | Tyrosine kinase receptor A (Trk-A) | Tyrosine kinase receptor A (TrkA) | gp140trk | p140-TrkA | ||
| Type: | n/a | ||
| Mol. Mass.: | 87498.18 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P04629 | ||
| Residue: | 796 | ||
| Sequence: |
| ||
| BDBM344324 | |||
| n/a | |||
| Name | BDBM344324 | ||
| Synonyms: | US10251889, Example 33 | US10758542, Example 33 | US9782415, Example 33 | US9796724, Example 33 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C24H25F2N5O3 | ||
| Mol. Mass. | 469.4838 | ||
| SMILES | OC(=O)[C@H]1CC[C@@H](CC1)NC(=O)c1cnn2ccc(nc12)N1CCC[C@@H]1c1cc(F)ccc1F |r,wU:6.9,wD:25.29,3.2,(4.87,-7.18,;5.96,-6.09,;7.45,-6.49,;5.57,-4.61,;6.64,-3.46,;6.24,-1.98,;4.75,-1.58,;3.66,-2.67,;4.06,-4.15,;4.35,-.09,;2.87,.31,;1.78,-.78,;2.47,1.8,;3.37,3.04,;2.47,4.29,;1,3.81,;-.33,4.58,;-1.66,3.81,;-1.66,2.27,;-.33,1.5,;1,2.27,;-3,1.5,;-3,-.04,;-4.46,-.51,;-5.37,.73,;-4.46,1.98,;-4.86,3.46,;-3.77,4.55,;-4.17,6.04,;-3.08,7.13,;-5.66,6.44,;-6.75,5.35,;-6.35,3.86,;-7.44,2.77,)| | ||
| Structure | 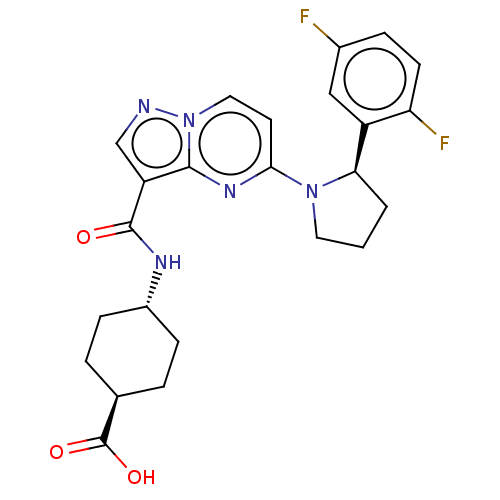
| ||