

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM85339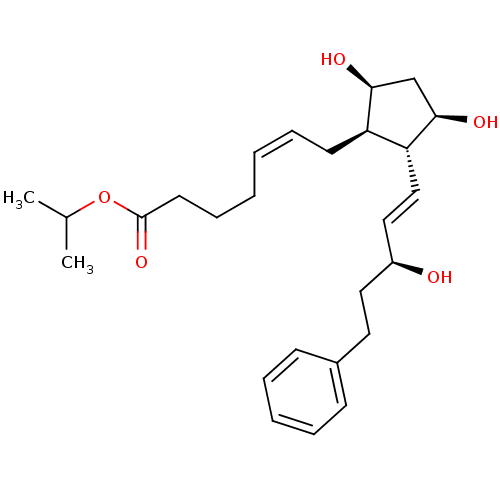 (17-PHENYL TRINOR PGF2ALPHA-IPR | CAS_130209-76-6 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 2A1 (Homo sapiens (Human)) | BDBM85720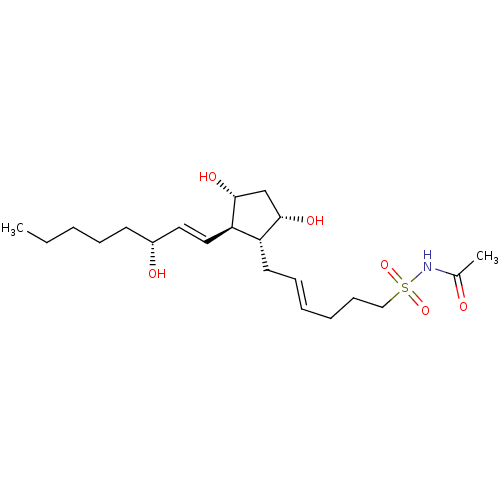 (AGN 194394) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50495950 (Danoprevir | R-05190591 | R05190591 | RO-5190591 |...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1a NS3/4A protease A156T mutant expressed in Escherichia coli BL21 (DE3) cells assessed as substrate cleavag... | J Med Chem 57: 1753-69 (2014) Article DOI: 10.1021/jm400164c BindingDB Entry DOI: 10.7270/Q2T72MD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 2A1 (Homo sapiens (Human)) | BDBM50020300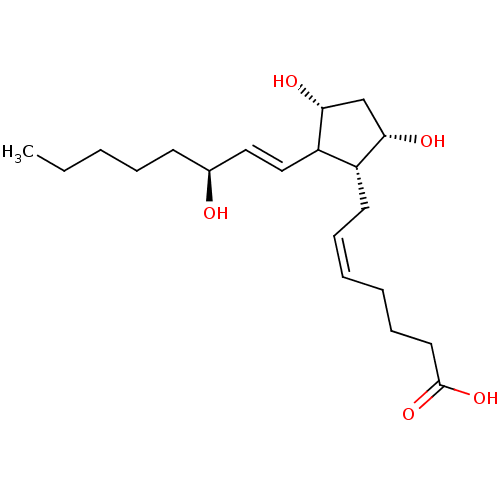 ((S-isomer)7-[3,5-Dihydroxy-2-(3-hydroxy-oct-1-enyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50020300 ((S-isomer)7-[3,5-Dihydroxy-2-(3-hydroxy-oct-1-enyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 2A1 (Homo sapiens (Human)) | BDBM85718 (AGN 191366) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 2A1 (Homo sapiens (Human)) | BDBM85339 (17-PHENYL TRINOR PGF2ALPHA-IPR | CAS_130209-76-6 |...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 168 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM85716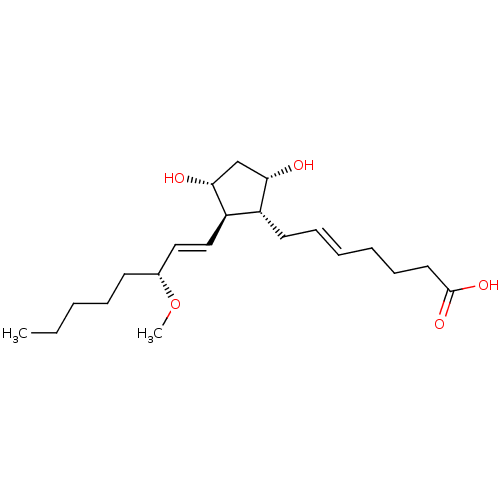 (AGN 191995) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 228 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 2A1 (Homo sapiens (Human)) | BDBM85715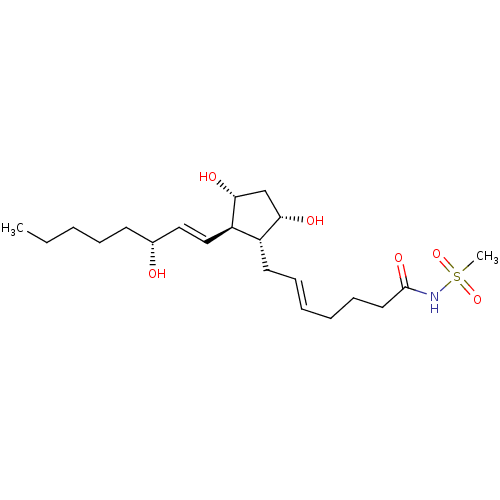 (AGN 191365) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 307 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM85719 (PGF2 Alpha, 1-isopropyl ester) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 2A1 (Homo sapiens (Human)) | BDBM85719 (PGF2 Alpha, 1-isopropyl ester) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 2A1 (Homo sapiens (Human)) | BDBM85714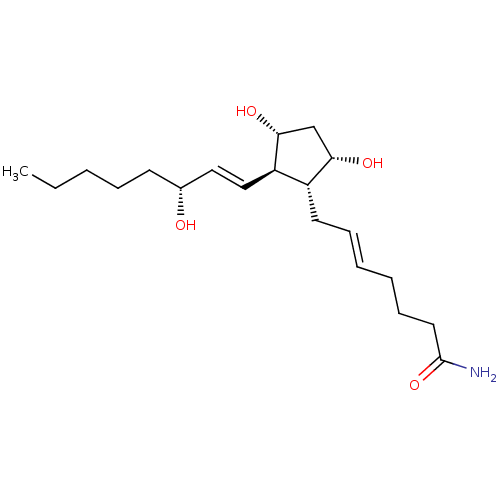 (AGN 190911) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM85718 (AGN 191366) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 2A1 (Homo sapiens (Human)) | BDBM85717 (AGN 192151) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM85720 (AGN 194394) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM85717 (AGN 192151) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 2A1 (Homo sapiens (Human)) | BDBM85721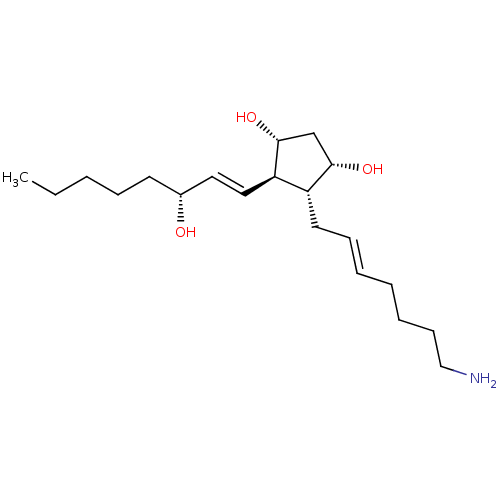 (AGN 191088) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM85715 (AGN 191365) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM85721 (AGN 191088) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM85714 (AGN 190911) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 2A1 (Homo sapiens (Human)) | BDBM85716 (AGN 191995) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by PDSP Ki Database | Mol Pharmacol 58: 1511-6 (2000) Article DOI: 10.1124/mol.58.6.1511 BindingDB Entry DOI: 10.7270/Q2WD3Z4B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM127782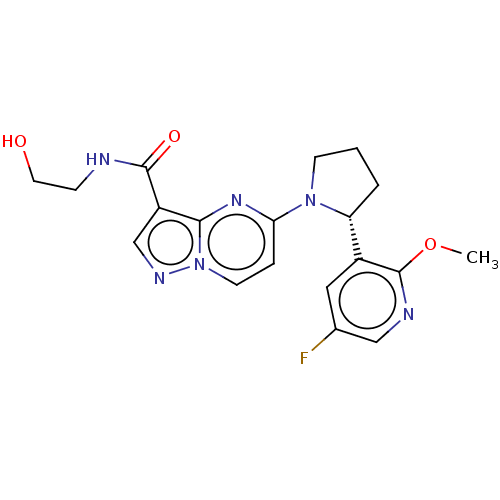 (US10251889, Example 155 | US10758542, Example 155 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description Compounds of Formula I were screened for their ability to inhibit Jak2 using the general enzyme inhibition assay method, in which the assay mixture c... | J Med Chem 50: 1876-85 (2007) BindingDB Entry DOI: 10.7270/Q2R49T22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM127782 (US10251889, Example 155 | US10758542, Example 155 ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma, Inc. US Patent | Assay Description Trk enzymatic selectivity was assessed using Omnia™ Kinase Assay reagents from Invitrogen Corp. Enzyme (either TrkA or TrkB from Invitrogen Corp.) an... | US Patent US9796724 (2017) BindingDB Entry DOI: 10.7270/Q27M0B21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS [G2032R] (Homo sapiens (Human)) | BDBM267453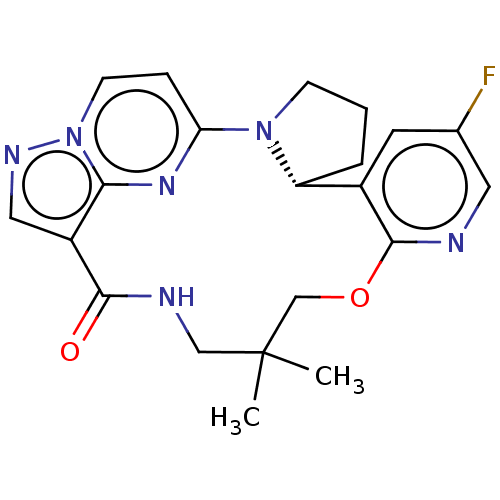 (US10688100, Compound 36 | US10966985, Compound 36 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description The potency of a compound inhibiting wild type and exemplary mutant ROS1 kinases was determined using CisBio's HTRF Kinease-TK assay technology. ... | US Patent US10688100 (2020) BindingDB Entry DOI: 10.7270/Q2KH0RC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM127782 (US10251889, Example 155 | US10758542, Example 155 ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma, Inc. US Patent | Assay Description An enzyme-linked immunosorbant assay (ELISA) was used to assess TrkA kinase activity in the presence of inhibitors. Immulon 4HBX 384-well microtiter ... | US Patent US9782415 (2017) BindingDB Entry DOI: 10.7270/Q2P84F0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM127782 (US10251889, Example 155 | US10758542, Example 155 ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Array Biopharma, Inc. US Patent | Assay Description An enzyme-linked immunosorbant assay (ELISA) was used to assess TrkA kinase activity in the presence of inhibitors. Immulon 4HBX 384-well microtiter ... | US Patent US8791123 (2014) BindingDB Entry DOI: 10.7270/Q2Q23XX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM127782 (US10251889, Example 155 | US10758542, Example 155 ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description An enzyme-linked immunosorbant assay (ELISA) was used to assess TrkA kinase activity in the presence of inhibitors. Immulon 4HBX 384-well microtiter ... | US Patent US10758542 (2020) BindingDB Entry DOI: 10.7270/Q2RB77PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS [D2033N] (Homo sapiens (Human)) | BDBM267453 (US10688100, Compound 36 | US10966985, Compound 36 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description The potency of a compound inhibiting wild type and exemplary mutant ROS1 kinases was determined using CisBio's HTRF Kinease-TK assay technology. ... | US Patent US10966985 (2021) BindingDB Entry DOI: 10.7270/Q2639ST6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS [D2033N] (Homo sapiens (Human)) | BDBM267407 (US10966985, Compound 3 | US9718822, 3 | US9750744,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description The potency of a compound inhibiting wild type and exemplary mutant ROS1 kinases was determined using CisBio's HTRF Kinease-TK assay technology. ... | US Patent US10966985 (2021) BindingDB Entry DOI: 10.7270/Q2639ST6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS [G2032R] (Homo sapiens (Human)) | BDBM446700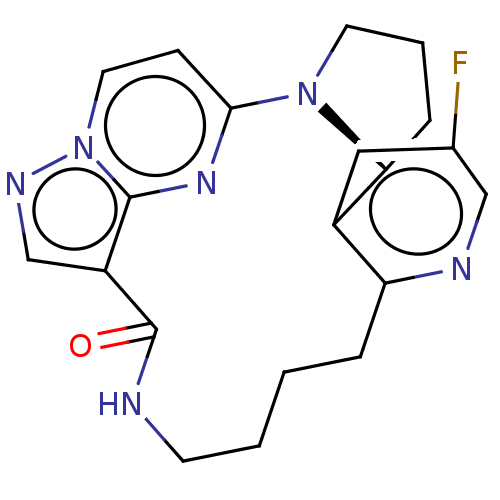 ((6R)-9-fluoro-13-oxa-2,11,17,21,22,25- hexaazapent...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description The potency of a compound inhibiting wild type and exemplary mutant ROS1 kinases was determined using CisBio's HTRF Kinease-TK assay technology. ... | US Patent US10688100 (2020) BindingDB Entry DOI: 10.7270/Q2KH0RC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM127652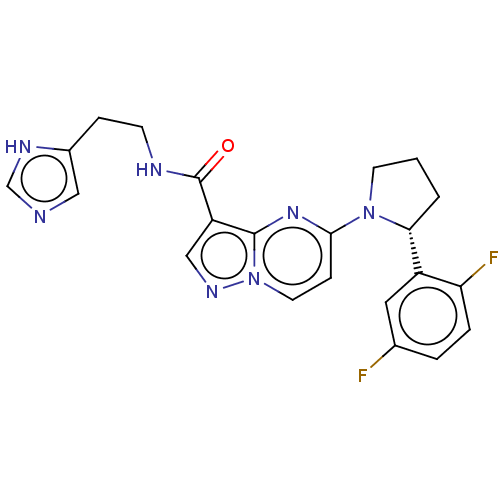 (US8791123, 25) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Array Biopharma, Inc. US Patent | Assay Description An enzyme-linked immunosorbant assay (ELISA) was used to assess TrkA kinase activity in the presence of inhibitors. Immulon 4HBX 384-well microtiter ... | US Patent US8791123 (2014) BindingDB Entry DOI: 10.7270/Q2Q23XX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM344316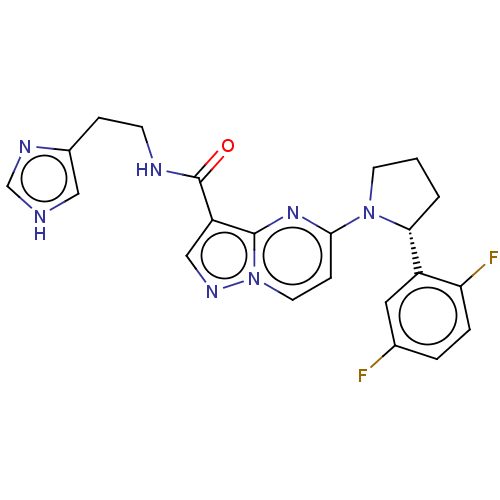 (US10251889, Example 25 | US10758542, Example 25 | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma, Inc. US Patent | Assay Description Trk enzymatic selectivity was assessed using Omnia™ Kinase Assay reagents from Invitrogen Corp. Enzyme (either TrkA or TrkB from Invitrogen Corp.) an... | US Patent US9796724 (2017) BindingDB Entry DOI: 10.7270/Q27M0B21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM344316 (US10251889, Example 25 | US10758542, Example 25 | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma, Inc. US Patent | Assay Description An enzyme-linked immunosorbant assay (ELISA) was used to assess TrkA kinase activity in the presence of inhibitors. Immulon 4HBX 384-well microtiter ... | US Patent US9782415 (2017) BindingDB Entry DOI: 10.7270/Q2P84F0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM344316 (US10251889, Example 25 | US10758542, Example 25 | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description An enzyme-linked immunosorbant assay (ELISA) was used to assess TrkA kinase activity in the presence of inhibitors. Immulon 4HBX 384-well microtiter ... | US Patent US10758542 (2020) BindingDB Entry DOI: 10.7270/Q2RB77PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM344316 (US10251889, Example 25 | US10758542, Example 25 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description Compounds of Formula I were screened for their ability to inhibit Jak2 using the general enzyme inhibition assay method, in which the assay mixture c... | J Med Chem 50: 1876-85 (2007) BindingDB Entry DOI: 10.7270/Q2R49T22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM127634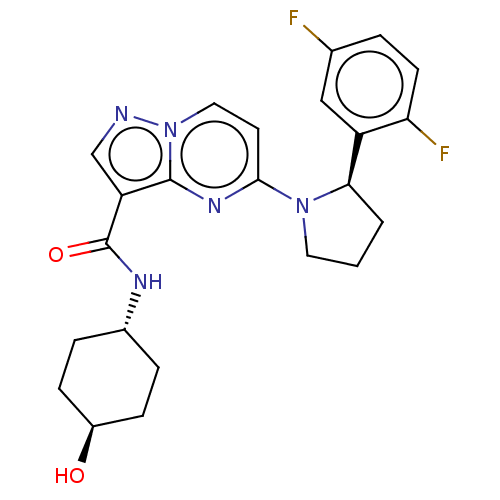 (US10251889, Example 7 | US10758542, Example 7 | US...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma, Inc. US Patent | Assay Description Trk enzymatic selectivity was assessed using Omnia™ Kinase Assay reagents from Invitrogen Corp. Enzyme (either TrkA or TrkB from Invitrogen Corp.) an... | US Patent US9796724 (2017) BindingDB Entry DOI: 10.7270/Q27M0B21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM127634 (US10251889, Example 7 | US10758542, Example 7 | US...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma, Inc. US Patent | Assay Description An enzyme-linked immunosorbant assay (ELISA) was used to assess TrkA kinase activity in the presence of inhibitors. Immulon 4HBX 384-well microtiter ... | US Patent US9782415 (2017) BindingDB Entry DOI: 10.7270/Q2P84F0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM127634 (US10251889, Example 7 | US10758542, Example 7 | US...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Array Biopharma, Inc. US Patent | Assay Description An enzyme-linked immunosorbant assay (ELISA) was used to assess TrkA kinase activity in the presence of inhibitors. Immulon 4HBX 384-well microtiter ... | US Patent US8791123 (2014) BindingDB Entry DOI: 10.7270/Q2Q23XX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM127634 (US10251889, Example 7 | US10758542, Example 7 | US...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description An enzyme-linked immunosorbant assay (ELISA) was used to assess TrkA kinase activity in the presence of inhibitors. Immulon 4HBX 384-well microtiter ... | US Patent US10758542 (2020) BindingDB Entry DOI: 10.7270/Q2RB77PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM127634 (US10251889, Example 7 | US10758542, Example 7 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description Compounds of Formula I were screened for their ability to inhibit Jak2 using the general enzyme inhibition assay method, in which the assay mixture c... | J Med Chem 50: 1876-85 (2007) BindingDB Entry DOI: 10.7270/Q2R49T22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM127640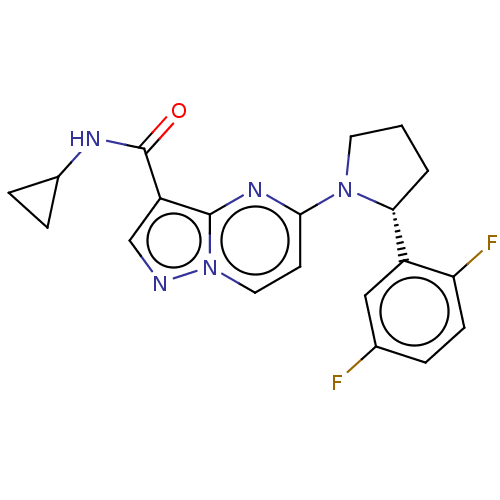 (US10251889, Example 13 | US10758542, Example 13 | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma, Inc. US Patent | Assay Description Trk enzymatic selectivity was assessed using Omnia™ Kinase Assay reagents from Invitrogen Corp. Enzyme (either TrkA or TrkB from Invitrogen Corp.) an... | US Patent US9796724 (2017) BindingDB Entry DOI: 10.7270/Q27M0B21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM127640 (US10251889, Example 13 | US10758542, Example 13 | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma, Inc. US Patent | Assay Description An enzyme-linked immunosorbant assay (ELISA) was used to assess TrkA kinase activity in the presence of inhibitors. Immulon 4HBX 384-well microtiter ... | US Patent US9782415 (2017) BindingDB Entry DOI: 10.7270/Q2P84F0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM127640 (US10251889, Example 13 | US10758542, Example 13 | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Array Biopharma, Inc. US Patent | Assay Description An enzyme-linked immunosorbant assay (ELISA) was used to assess TrkA kinase activity in the presence of inhibitors. Immulon 4HBX 384-well microtiter ... | US Patent US8791123 (2014) BindingDB Entry DOI: 10.7270/Q2Q23XX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS [D2033N] (Homo sapiens (Human)) | BDBM50507492 (Loxo-195 | Selitrectinib | US10966985, Compound 33...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description The potency of a compound inhibiting wild type and exemplary mutant ROS1 kinases was determined using CisBio's HTRF Kinease-TK assay technology. ... | US Patent US10966985 (2021) BindingDB Entry DOI: 10.7270/Q2639ST6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM127640 (US10251889, Example 13 | US10758542, Example 13 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description Compounds of Formula I were screened for their ability to inhibit Jak2 using the general enzyme inhibition assay method, in which the assay mixture c... | J Med Chem 50: 1876-85 (2007) BindingDB Entry DOI: 10.7270/Q2R49T22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM127640 (US10251889, Example 13 | US10758542, Example 13 | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description An enzyme-linked immunosorbant assay (ELISA) was used to assess TrkA kinase activity in the presence of inhibitors. Immulon 4HBX 384-well microtiter ... | US Patent US10758542 (2020) BindingDB Entry DOI: 10.7270/Q2RB77PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS [G2032R] (Homo sapiens (Human)) | BDBM50507492 (Loxo-195 | Selitrectinib | US10966985, Compound 33...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description The potency of a compound inhibiting wild type and exemplary mutant ROS1 kinases was determined using CisBio's HTRF Kinease-TK assay technology. ... | US Patent US10688100 (2020) BindingDB Entry DOI: 10.7270/Q2KH0RC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS [D2033N] (Homo sapiens (Human)) | BDBM267453 (US10688100, Compound 36 | US10966985, Compound 36 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description The potency of a compound inhibiting wild type and exemplary mutant ROS1 kinases was determined using CisBio's HTRF Kinease-TK assay technology. ... | US Patent US10688100 (2020) BindingDB Entry DOI: 10.7270/Q2KH0RC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS [L2026M] (Homo sapiens (Human)) | BDBM267453 (US10688100, Compound 36 | US10966985, Compound 36 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description The potency of a compound inhibiting wild type and exemplary mutant ROS1 kinases was determined using CisBio's HTRF Kinease-TK assay technology. ... | US Patent US10966985 (2021) BindingDB Entry DOI: 10.7270/Q2639ST6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM136465 (US8865726, 96) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Array BioPharma Inc. US Patent | Assay Description The ability of compounds of Formula I to inhibit mTOR was determined in a radioactive filtration assay that measures the transfer of radiolabeled pho... | US Patent US8865726 (2014) BindingDB Entry DOI: 10.7270/Q2V69H93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 26382 total ) | Next | Last >> |