

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | RAC-alpha serine/threonine-protein kinase | ||
| Ligand | BDBM50528435 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_1904982 (CHEMBL4407340) | ||
| IC50 | 3.8±n/a nM | ||
| Citation |  Dong, X; Zhan, W; Zhao, M; Che, J; Dai, X; Wu, Y; Xu, L; Zhou, Y; Zhao, Y; Tian, T; Cheng, G; Jin, Z; Li, J; Shao, Y; He, Q; Yang, B; Weng, Q; Hu, Y Discovery of 3,4,6-Trisubstituted Piperidine Derivatives as Orally Active, Low hERG Blocking Akt Inhibitors via Conformational Restriction and Structure-Based Design. J Med Chem62:7264-7288 (2019) [PubMed] Article Dong, X; Zhan, W; Zhao, M; Che, J; Dai, X; Wu, Y; Xu, L; Zhou, Y; Zhao, Y; Tian, T; Cheng, G; Jin, Z; Li, J; Shao, Y; He, Q; Yang, B; Weng, Q; Hu, Y Discovery of 3,4,6-Trisubstituted Piperidine Derivatives as Orally Active, Low hERG Blocking Akt Inhibitors via Conformational Restriction and Structure-Based Design. J Med Chem62:7264-7288 (2019) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| RAC-alpha serine/threonine-protein kinase | |||
| Name: | RAC-alpha serine/threonine-protein kinase | ||
| Synonyms: | AKT phosphorylation (p-AKT) | AKT1 | AKT1/PPP1CA | AKT1_HUMAN | C-AKT | PKB | PKB alpha | Protein kinase Akt-1 | Protein kinase B | Protein kinase B (AKT1) | Protein kinase B (Akt 1) | Protein kinase B (Akt) | Protein kinase B alpha | Protein kinase B alpha (AKT1) | Proto-oncogene Akt (Akt1) | Proto-oncogene c-Akt (AKT) | Proto-oncogene c-Akt (AKT1) | RAC | RAC-PK-alpha | RAC-alpha serine/threonine-protein kinase (AKT) | RAC-alpha serine/threonine-protein kinase (AKT1) | RAC-alpha serine/threonine-protein kinase (pAKT) | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 55681.25 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P31749 | ||
| Residue: | 480 | ||
| Sequence: |
| ||
| BDBM50528435 | |||
| n/a | |||
| Name | BDBM50528435 | ||
| Synonyms: | CHEMBL4470606 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C23H24Cl4N4O4 | ||
| Mol. Mass. | 562.273 | ||
| SMILES | Cn1ncc(Cl)c1-c1cc(oc1Cl)C(=O)N[C@@H]1CN[C@H](CC(O)CO)C[C@H]1c1ccc(Cl)c(Cl)c1 |r,wU:16.17,19.21,wD:26.29,(6.28,-22.36,;5.95,-23.87,;4.54,-24.49,;4.7,-26.02,;6.21,-26.34,;6.83,-27.75,;6.98,-25.01,;8.52,-24.85,;9.54,-26,;10.96,-25.38,;10.79,-23.85,;9.29,-23.52,;8.67,-22.11,;12.29,-26.15,;12.29,-27.69,;13.63,-25.38,;14.96,-26.16,;14.95,-27.69,;16.28,-28.47,;17.62,-27.7,;18.95,-28.48,;20.29,-27.71,;20.29,-26.17,;21.62,-28.49,;22.96,-27.72,;17.63,-26.16,;16.29,-25.39,;16.29,-23.86,;14.96,-23.09,;14.96,-21.54,;16.29,-20.77,;16.29,-19.23,;17.63,-21.54,;18.96,-20.76,;17.63,-23.09,)| | ||
| Structure | 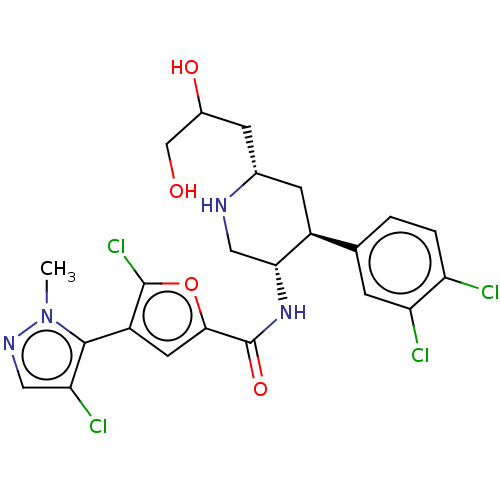
| ||