

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479434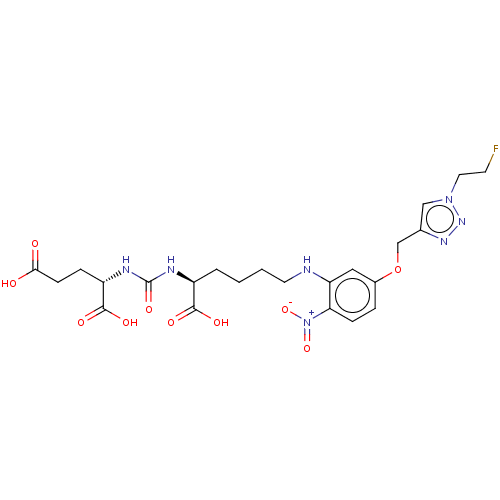 (US10894807, ID P242) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50502477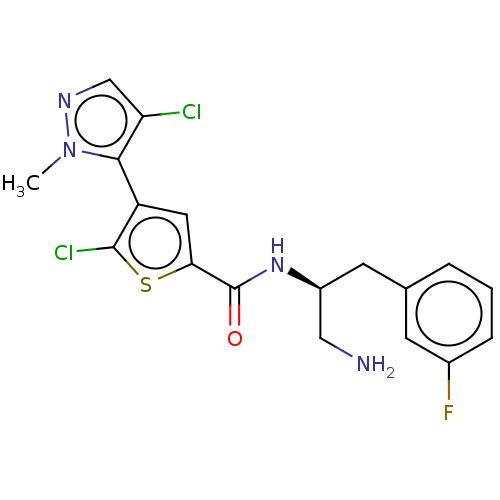 (ASB-183 | ASB183 | Afuresertib | GSK-2110183C | GS...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Institute of Innovative Medicine Curated by ChEMBL | Assay Description Inhibition of Akt1 (unknown origin) | Eur J Med Chem 180: 72-85 (2019) Article DOI: 10.1016/j.ejmech.2019.07.017 BindingDB Entry DOI: 10.7270/Q2Q243H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50091350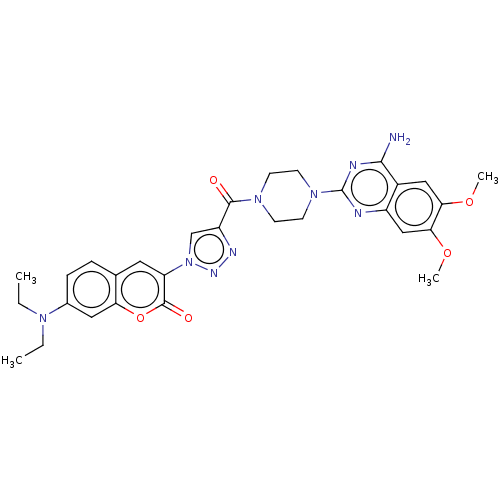 (CHEMBL3582270) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Displacement of [3H]-Prazosin from human alpha-1B adrenergic receptor transfected in CHO cell membranes after 2 hrs by microplate scintillation count... | ACS Med Chem Lett 6: 502-6 (2015) Article DOI: 10.1021/ml5004298 BindingDB Entry DOI: 10.7270/Q2VM4DZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha1-adrenoceptor (unknown origin) in cerebral cortex membranes after 60 mins by TopCount scintillation counting ... | Eur J Med Chem 144: 701-715 (2018) Article DOI: 10.1016/j.ejmech.2017.12.063 BindingDB Entry DOI: 10.7270/Q26D5WP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479437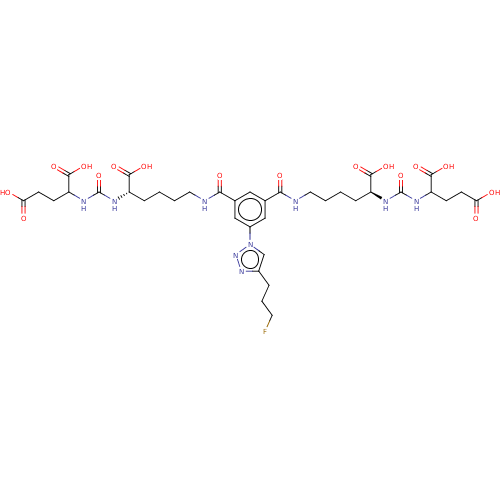 (US10894807, ID P246) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM497267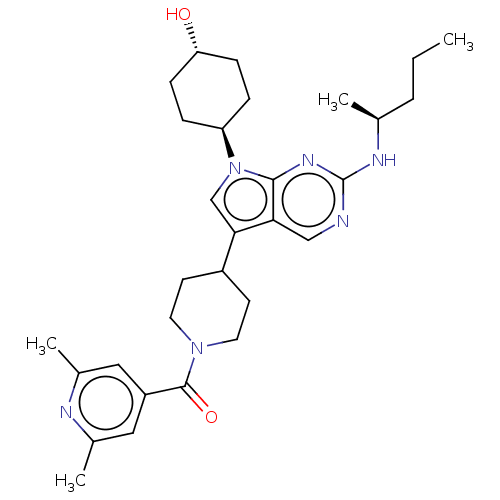 ((2,6-dimethylpyridin-4- yl)(4-(7-((1R,4S)-4- hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATP competitive inhibition of MERTK (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113534 BindingDB Entry DOI: 10.7270/Q2M90DGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479444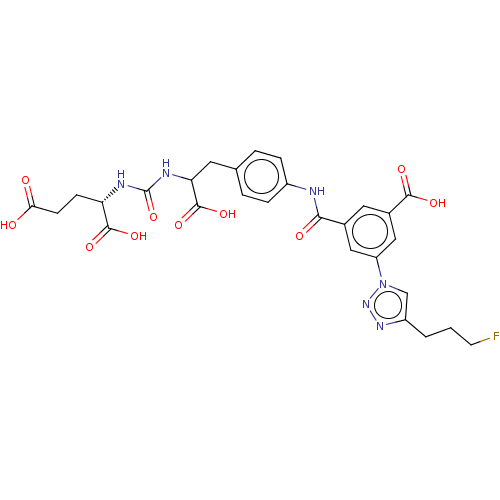 (US10894807, ID P253) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479450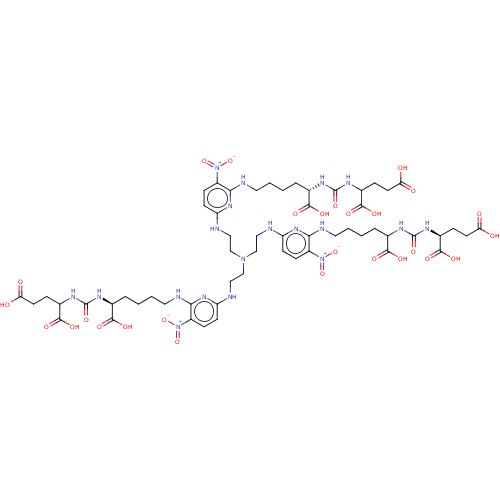 (US10894807, ID P270) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479453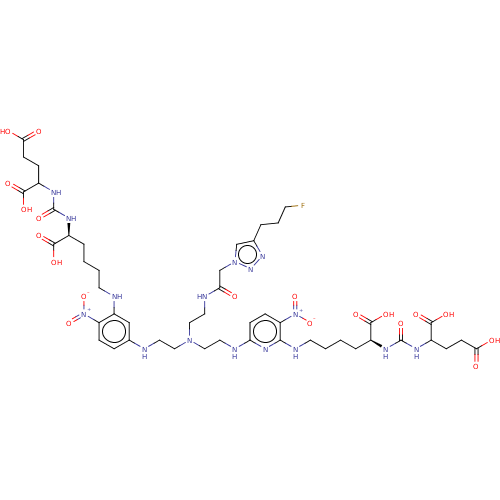 (US10894807, ID P273) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50151982 (5-{4-[4-(5-Cyano-1H-indol-3-yl)-butyl]-piperazin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China State Institute of Pharmaceutical Industry Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5HT1A receptor (unknown origin) expressed in HEK293 cells membranes incubated for 60 mins by scintillation counti... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126703 BindingDB Entry DOI: 10.7270/Q2DR2ZZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479458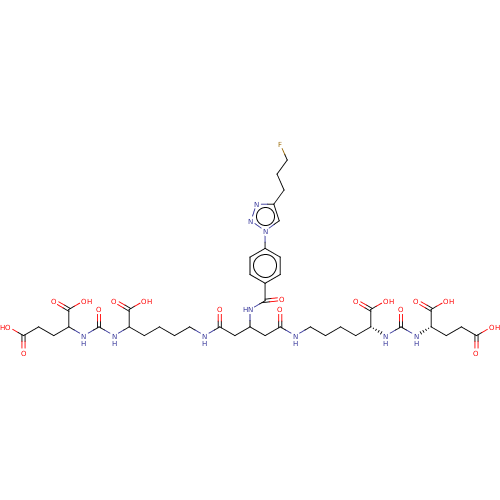 (US10894807, ID P278) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50579244 (Avoralstat | BCX-4161) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of purified human plasma kallikrein assessed as inhibition constant using H-D-Pro-Phe-Arg-pNA.2HCl as substrate measured after 3 mins by m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00511 BindingDB Entry DOI: 10.7270/Q29C7288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50464861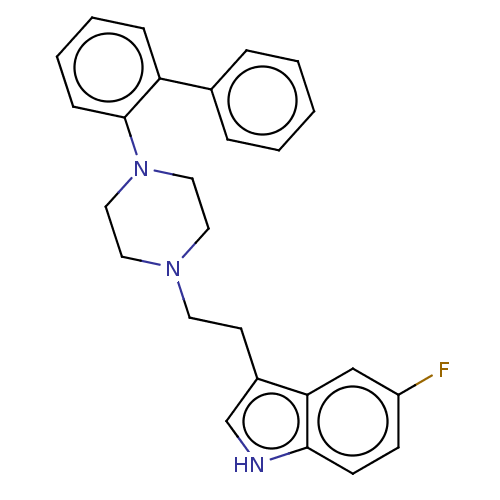 (CHEMBL4291322) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry Curated by ChEMBL | Assay Description Displacement of [3H]LSD from recombinant human 5-HT7 receptor expressed in CHO cell membranes after 120 mins by TopCount scintillation counting metho... | Eur J Med Chem 144: 701-715 (2018) Article DOI: 10.1016/j.ejmech.2017.12.063 BindingDB Entry DOI: 10.7270/Q26D5WP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479429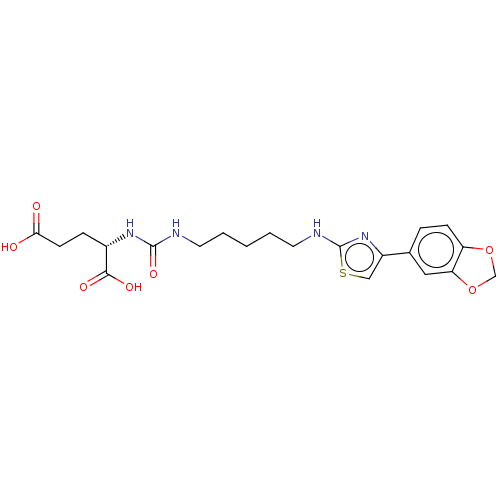 (US10894807, ID P235) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50532486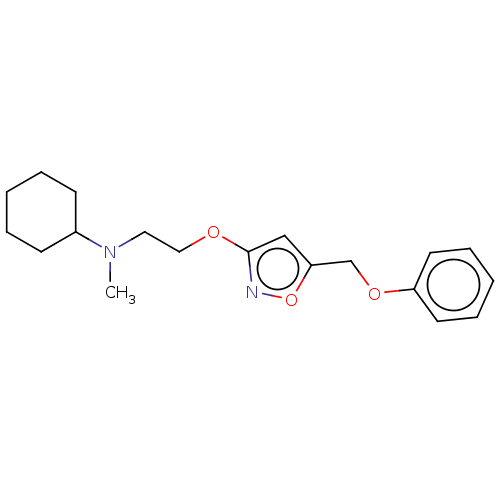 (CHEMBL4564992) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocine from rat brain sigma1 receptor by PDSP assay | J Med Chem 59: 6329-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00571 BindingDB Entry DOI: 10.7270/Q2K93C14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50091350 (CHEMBL3582270) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Displacement of [3H]-Prazosin from human alpha-1A adrenergic receptor transfected in CHO cell membranes after 2 hrs by microplate scintillation count... | ACS Med Chem Lett 6: 502-6 (2015) Article DOI: 10.1021/ml5004298 BindingDB Entry DOI: 10.7270/Q2VM4DZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479449 (US10894807, ID P266) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479415 (US10894807, ID P200) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50532486 (CHEMBL4564992) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocine from rat brain sigma1 receptor by PDSP assay | J Med Chem 59: 6329-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00571 BindingDB Entry DOI: 10.7270/Q2K93C14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479431 (US10894807, ID P238) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]ketanserine from 5-HT2A receptor in rat cerebral cortex homogenates after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 25: 5299-305 (2015) Article DOI: 10.1016/j.bmcl.2015.09.045 BindingDB Entry DOI: 10.7270/Q26W9CW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50055499 (CHEMBL3326002) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer (unknown origin) by Off-chip Mobility Shift Assay | J Med Chem 57: 7031-41 (2014) Article DOI: 10.1021/jm500749d BindingDB Entry DOI: 10.7270/Q2K075XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479442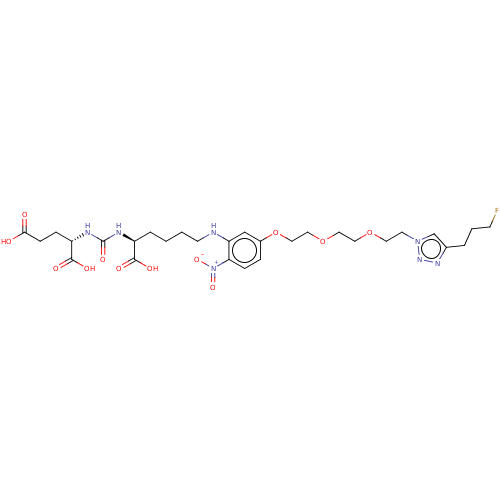 (US10894807, ID P251) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479435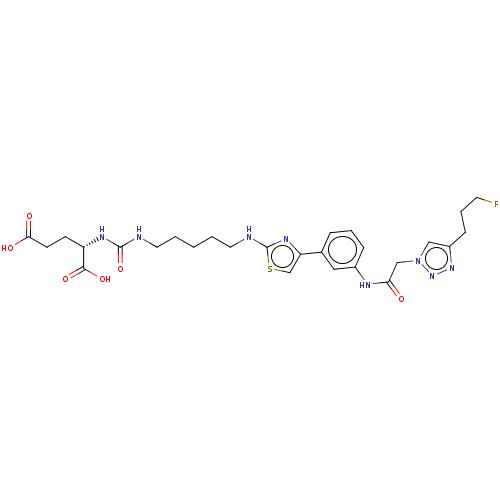 (US10894807, ID P244) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479433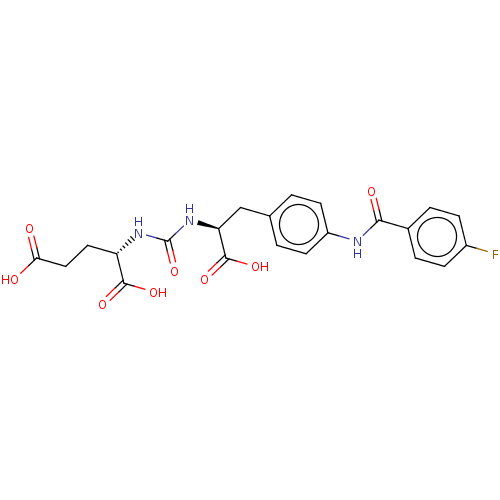 (US10894807, ID P241) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479445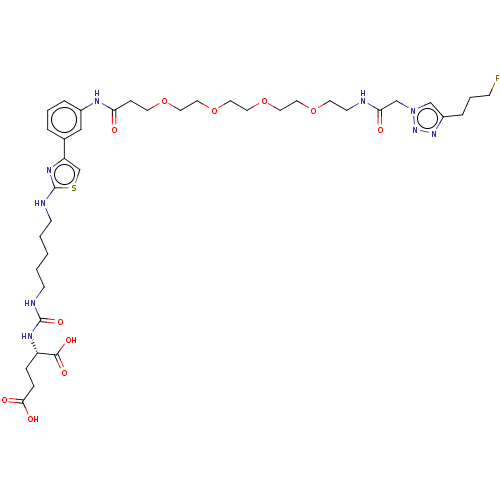 (US10894807, ID P254) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50091350 (CHEMBL3582270) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Displacement of [3H]-Prazosin from human alpha-1D adrenergic receptor transfected in CHO cell membranes after 2 hrs by microplate scintillation count... | ACS Med Chem Lett 6: 502-6 (2015) Article DOI: 10.1021/ml5004298 BindingDB Entry DOI: 10.7270/Q2VM4DZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479457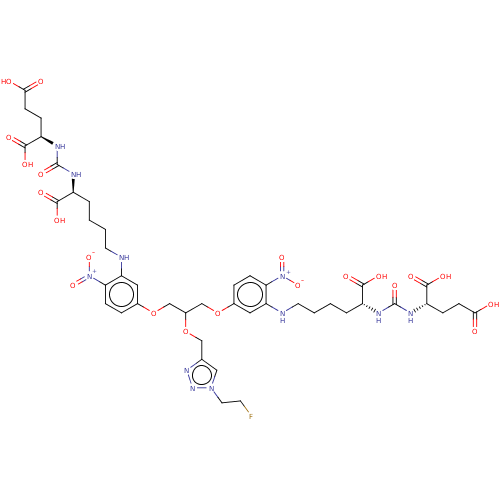 (US10894807, ID P277) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50469353 (CHEMBL4283353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNC Eshelman School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of MERTK (unknown origin) using 5'-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by MCE assay | J Med Chem 61: 10242-10254 (2018) Article DOI: 10.1021/acs.jmedchem.8b01229 BindingDB Entry DOI: 10.7270/Q2K076ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM416926 ((+)-1-(3-(aminomethyl)phenyl)-N-(5-((3-cyanophenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of purified human plasma kallikrein assessed as inhibition constant using H-D-Pro-Phe-Arg-pNA.2HCl as substrate measured after 3 mins by m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00511 BindingDB Entry DOI: 10.7270/Q29C7288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50150105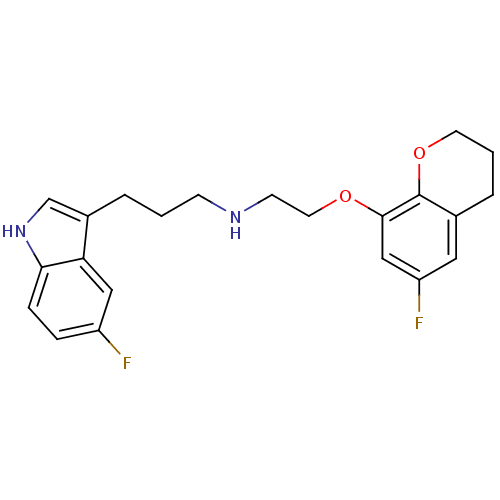 (CHEMBL124069 | [2-(6-Fluoro-chroman-8-yloxy)-ethyl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry Curated by ChEMBL | Assay Description Inhibition of serotonin reuptake in SERT (unknown origin) | Eur J Med Chem 144: 701-715 (2018) Article DOI: 10.1016/j.ejmech.2017.12.063 BindingDB Entry DOI: 10.7270/Q26D5WP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50532508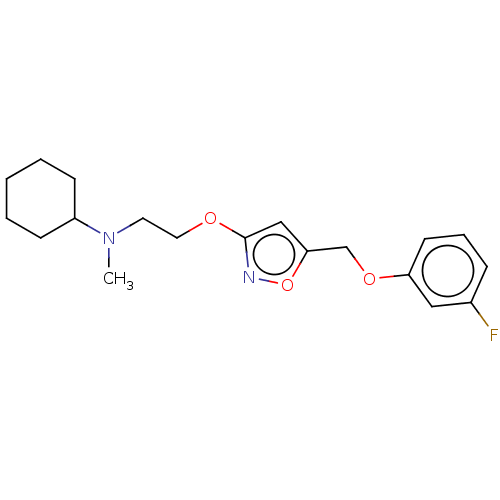 (CHEMBL4471576) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocine from rat brain sigma1 receptor by PDSP assay | J Med Chem 59: 6329-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00571 BindingDB Entry DOI: 10.7270/Q2K93C14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50532518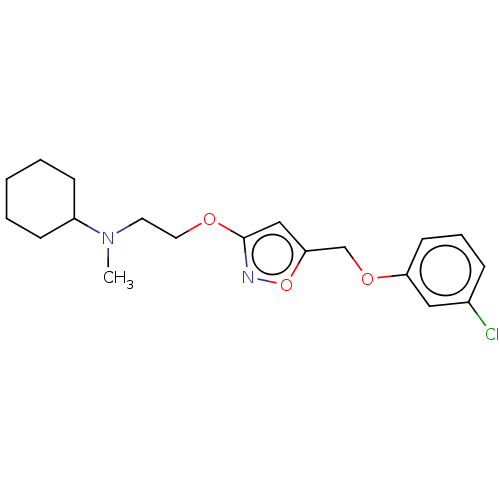 (CHEMBL4440420) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocine from rat brain sigma1 receptor by PDSP assay | J Med Chem 59: 6329-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00571 BindingDB Entry DOI: 10.7270/Q2K93C14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479464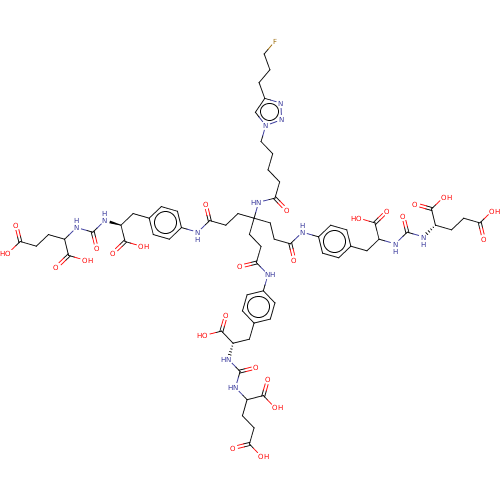 (US10894807, ID P285) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50532508 (CHEMBL4471576) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocine from rat brain sigma1 receptor by PDSP assay | J Med Chem 59: 6329-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00571 BindingDB Entry DOI: 10.7270/Q2K93C14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50532506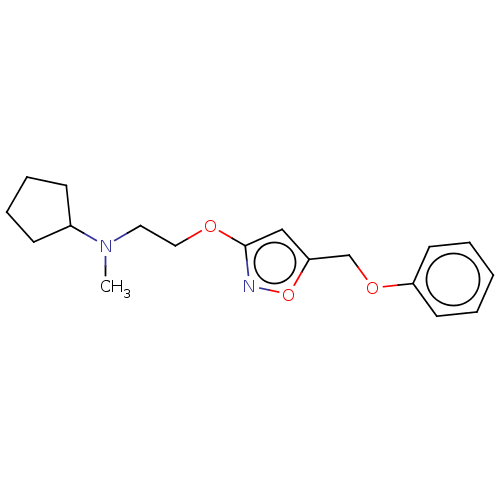 (CHEMBL4536431) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocine from rat brain sigma1 receptor by PDSP assay | J Med Chem 59: 6329-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00571 BindingDB Entry DOI: 10.7270/Q2K93C14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50532518 (CHEMBL4440420) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocine from rat brain sigma1 receptor by PDSP assay | J Med Chem 59: 6329-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00571 BindingDB Entry DOI: 10.7270/Q2K93C14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50532506 (CHEMBL4536431) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocine from rat brain sigma1 receptor by PDSP assay | J Med Chem 59: 6329-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00571 BindingDB Entry DOI: 10.7270/Q2K93C14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50464861 (CHEMBL4291322) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from recombinant human 5-HT1A receptor expressed in HEK293 cell membranes after 60 mins by TopCount scintillation count... | Eur J Med Chem 144: 701-715 (2018) Article DOI: 10.1016/j.ejmech.2017.12.063 BindingDB Entry DOI: 10.7270/Q26D5WP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50464857 (CHEMBL4277476) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry Curated by ChEMBL | Assay Description Displacement of [3H]LSD from recombinant human 5-HT7 receptor expressed in CHO cell membranes after 120 mins by TopCount scintillation counting metho... | Eur J Med Chem 144: 701-715 (2018) Article DOI: 10.1016/j.ejmech.2017.12.063 BindingDB Entry DOI: 10.7270/Q26D5WP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479426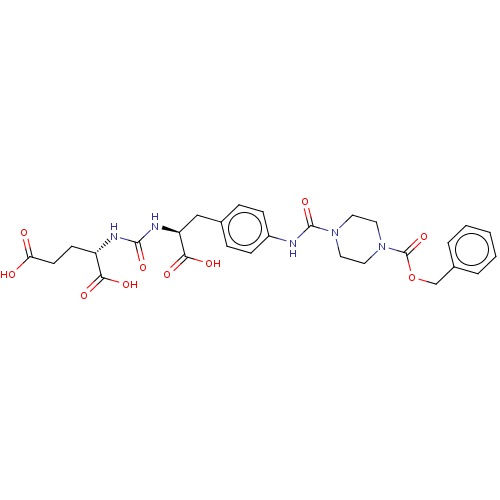 (US10894807, ID P222) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM31046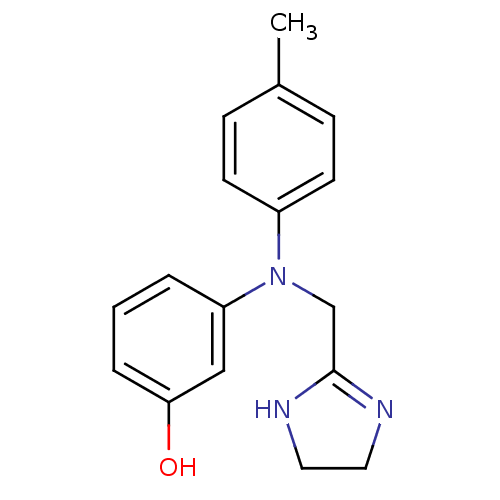 (3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Displacement of [3H]-Prazosin from human alpha-1A adrenergic receptor transfected in CHO cell membranes after 2 hrs by microplate scintillation count... | ACS Med Chem Lett 6: 502-6 (2015) Article DOI: 10.1021/ml5004298 BindingDB Entry DOI: 10.7270/Q2VM4DZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50225074 ((1S,3S,5S)-2-[(S)-2-amino-2-(3-hydroxy-adamantan-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of DPP4 | Eur J Med Chem 44: 3318-22 (2009) Article DOI: 10.1016/j.ejmech.2009.03.021 BindingDB Entry DOI: 10.7270/Q2DR2WGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479425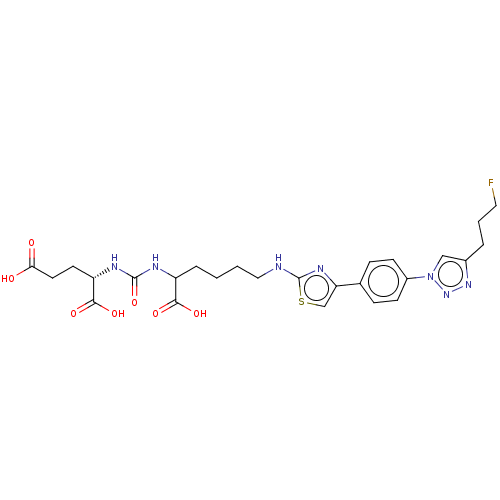 (US10894807, ID P218) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479438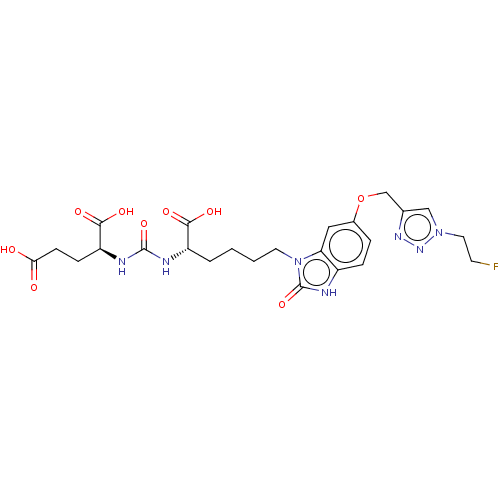 (US10894807, ID P247) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50532502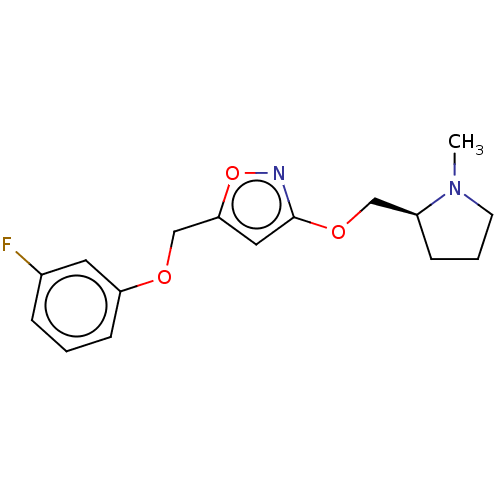 (CHEMBL4538923) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocine from rat brain sigma1 receptor by PDSP assay | J Med Chem 59: 6329-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00571 BindingDB Entry DOI: 10.7270/Q2K93C14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479469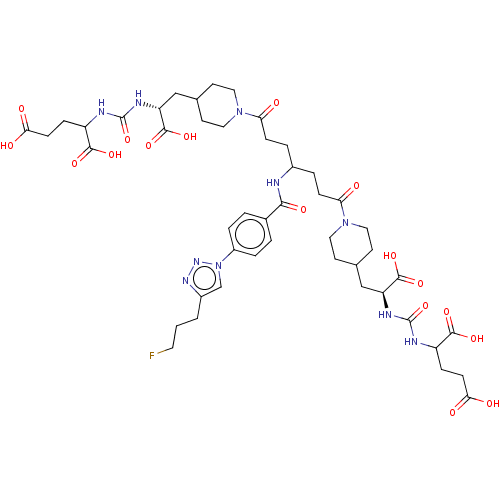 (US10894807, ID P292) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50532502 (CHEMBL4538923) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocine from rat brain sigma1 receptor by PDSP assay | J Med Chem 59: 6329-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00571 BindingDB Entry DOI: 10.7270/Q2K93C14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50193709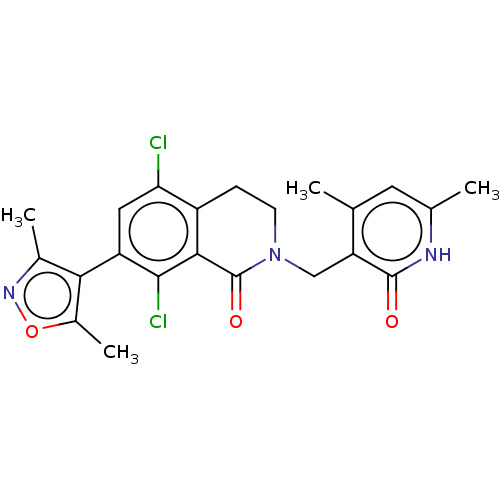 (CHEMBL3911017) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... | J Med Chem 59: 8306-25 (2016) Article DOI: 10.1021/acs.jmedchem.6b00515 BindingDB Entry DOI: 10.7270/Q2J1053B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50481120 (Flutemetamol | [3H]-Flutemetamol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity to Beta amyloid aggregates in Alzheimer's disease patient brain by competitive binding assay | J Med Chem 53: 933-41 (2010) Article DOI: 10.1021/jm901039z BindingDB Entry DOI: 10.7270/Q2RX9FW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 21980 total ) | Next | Last >> |