

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50577958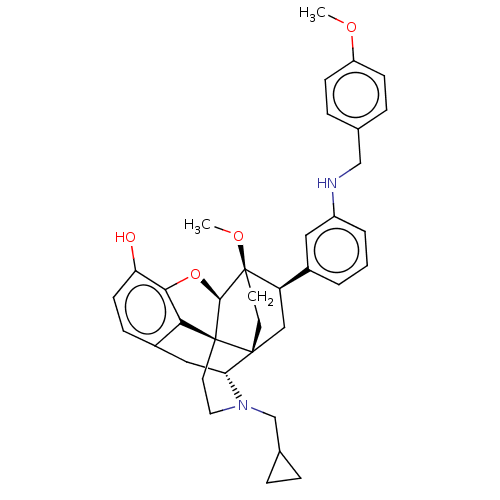 (CHEMBL4859512) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01082 BindingDB Entry DOI: 10.7270/Q2NZ8CG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082811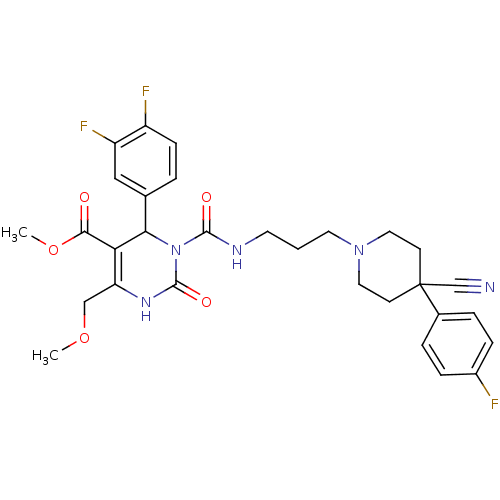 (3-{3-[4-Cyano-4-(4-fluoro-phenyl)-piperidin-1-yl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082827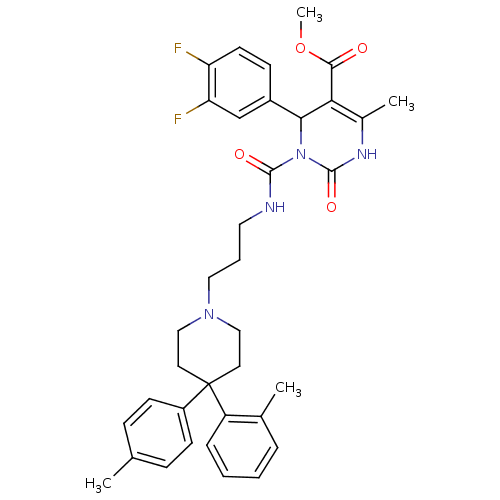 (4-(3,4-Difluoro-phenyl)-6-methyl-2-oxo-3-[3-(4-o-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50577958 (CHEMBL4859512) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPDPE from rat delta opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting metho... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01082 BindingDB Entry DOI: 10.7270/Q2NZ8CG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50567169 (CHEMBL4873876) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Apelin-13 from human APJ receptor stably expressed in human HEK293 cell membrane incubated for 120 mins by TopCount scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01878 BindingDB Entry DOI: 10.7270/Q20G3PXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082797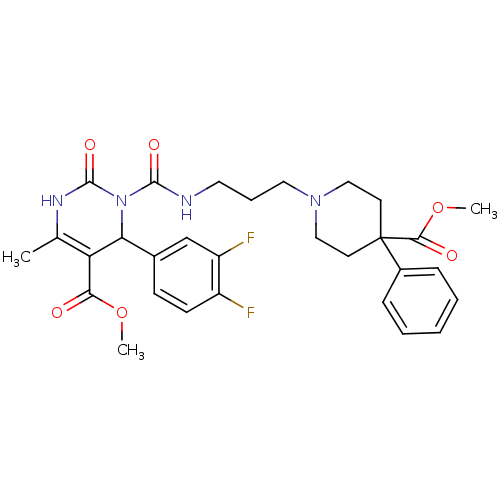 (4-(3,4-Difluoro-phenyl)-3-[3-(4-methoxycarbonyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082810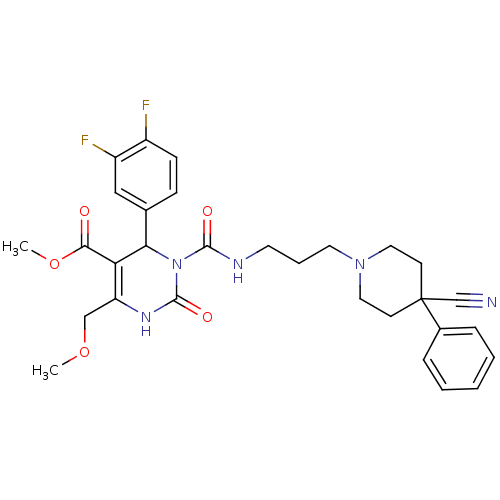 (3-[3-(4-Cyano-4-phenyl-piperidin-1-yl)-propylcarba...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082825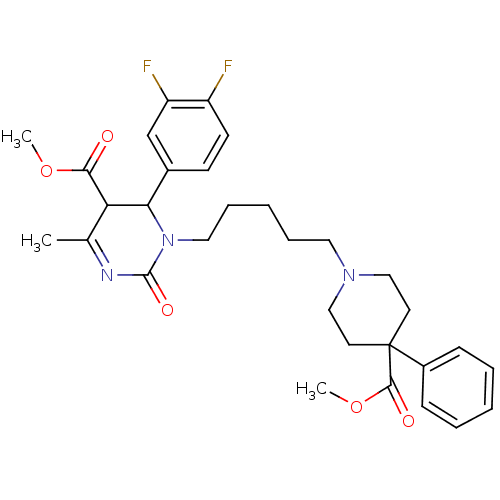 (4-(3,4-Difluoro-phenyl)-3-[5-(4-methoxycarbonyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133053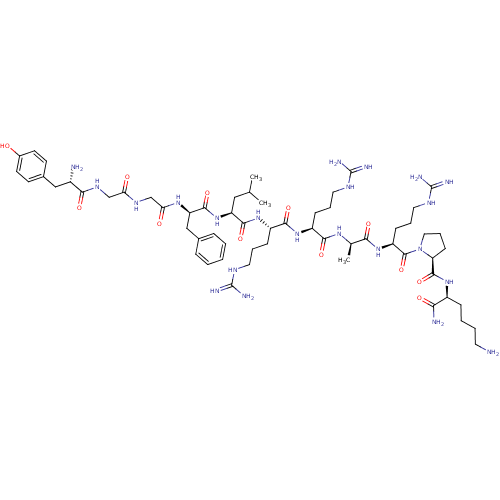 (CHEMBL405057 | Opioid Peptide [d-Ala(8)]Dynorphin ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity of the compound towards rat Opioid receptor kappa 1 expressed in CHO cells of chinese hamster ovary was determined using [3H]-dipren... | J Med Chem 46: 4002-8 (2003) Article DOI: 10.1021/jm030075o BindingDB Entry DOI: 10.7270/Q2CN739H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM85780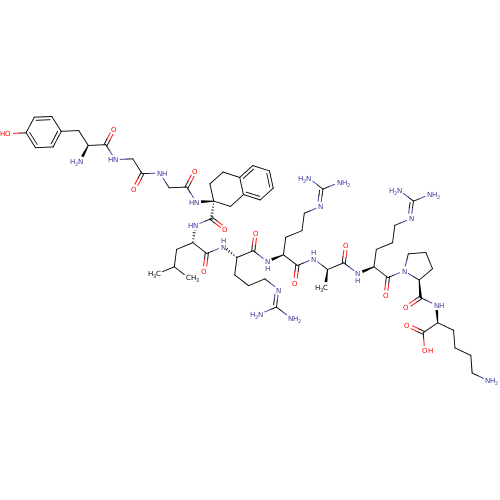 ([(R)-Atc4, D-Ala8]Dyn A-(1-11)NH2 | [(S)-Atc4 , D-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by PDSP Ki Database | Chirality 13: 125-9 (2001) Article DOI: 10.1002/1520-636X(2001)13:3 BindingDB Entry DOI: 10.7270/Q2SJ1J5H | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apelin receptor (Homo sapiens (Human)) | BDBM50014619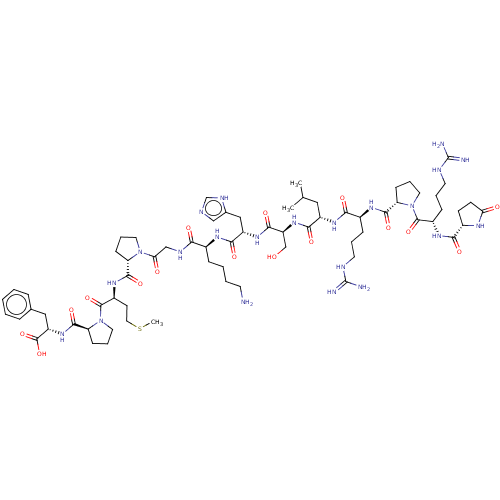 (CHEMBL3184840) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]Apelin-13 from human APJ receptor stably expressed in human HEK293 cell membrane incubated for 120 mins by TopCount scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01878 BindingDB Entry DOI: 10.7270/Q20G3PXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090647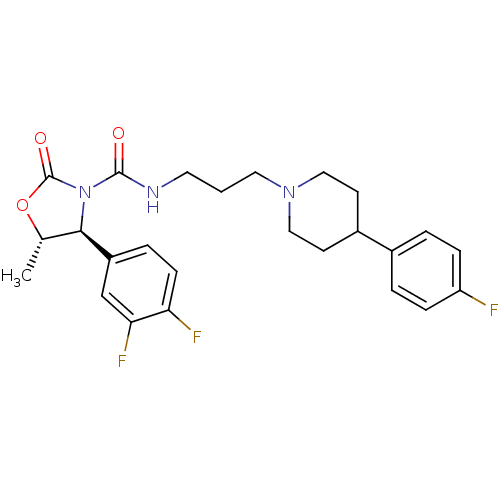 ((4S,5S)-4-(3,4-Difluoro-phenyl)-5-methyl-2-oxo-oxa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity of the compound towards Alpha1A human adrenergic receptors, using [125I]-HEAT as radioligand. | J Med Chem 43: 2775-8 (2000) BindingDB Entry DOI: 10.7270/Q2PZ582K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082819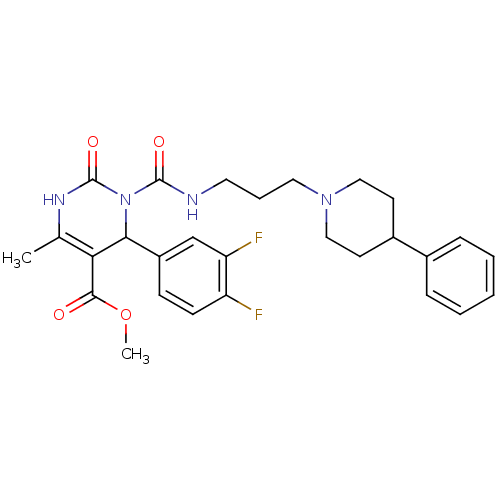 (4-(3,4-Difluoro-phenyl)-6-methyl-2-oxo-3-[3-(4-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082794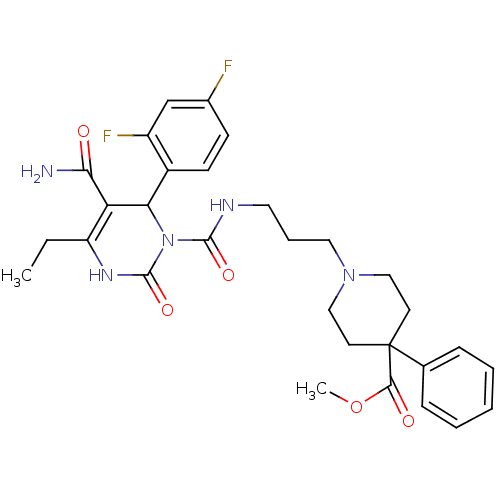 (1-(3-{[5-Carbamoyl-6-(2,4-difluoro-phenyl)-4-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082814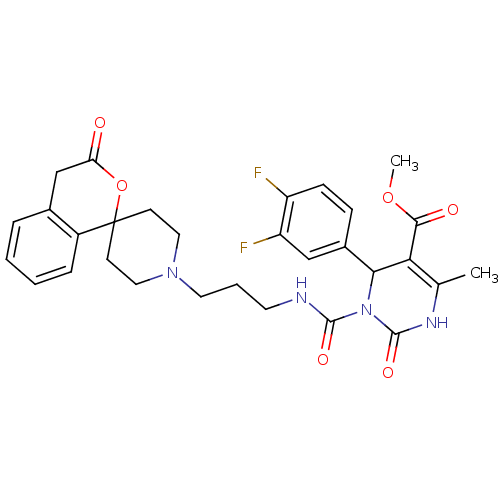 (CHEMBL358785 | methyl 4-(3,4-difluorophenyl)-6-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082821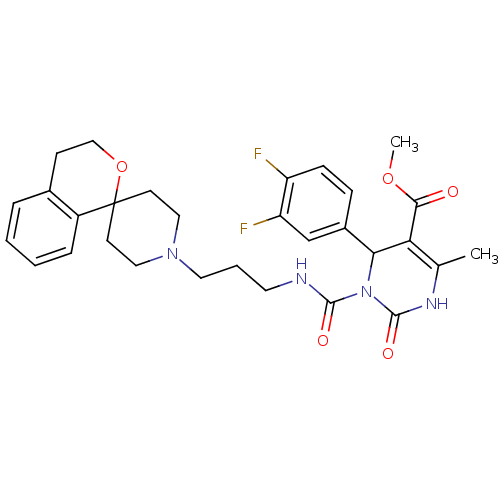 (CHEMBL359012 | methyl 4-(3,4-difluorophenyl)-6-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082813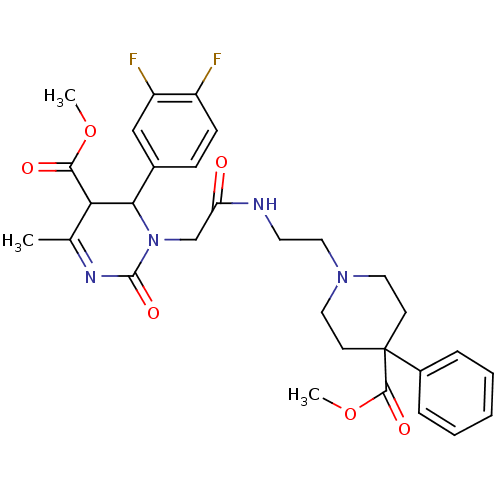 (4-(3,4-Difluoro-phenyl)-3-{[2-(4-methoxycarbonyl-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082784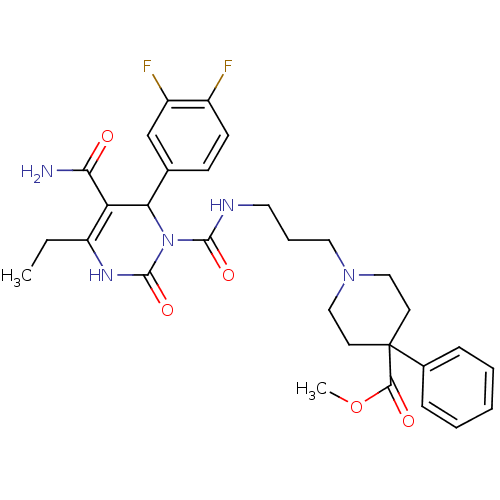 (1-(3-{[5-Carbamoyl-6-(3,4-difluoro-phenyl)-4-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082812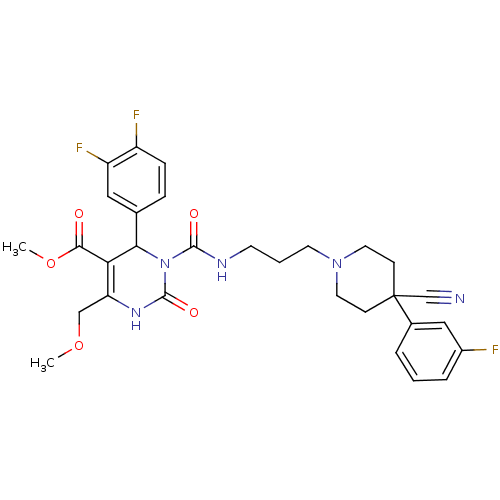 (3-{3-[4-Cyano-4-(3-fluoro-phenyl)-piperidin-1-yl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082835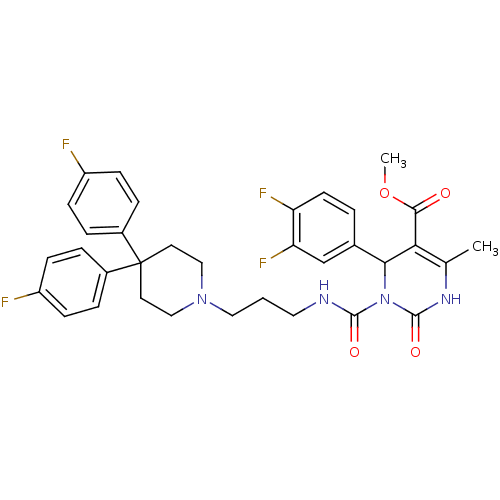 (3-{3-[4,4-Bis-(4-fluoro-phenyl)-piperidin-1-yl]-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090647 ((4S,5S)-4-(3,4-Difluoro-phenyl)-5-methyl-2-oxo-oxa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity towards Alpha1A dog adrenergic receptors, using [125I]HEAT as radioligand. | J Med Chem 43: 2775-8 (2000) BindingDB Entry DOI: 10.7270/Q2PZ582K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50577958 (CHEMBL4859512) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.294 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01082 BindingDB Entry DOI: 10.7270/Q2NZ8CG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082826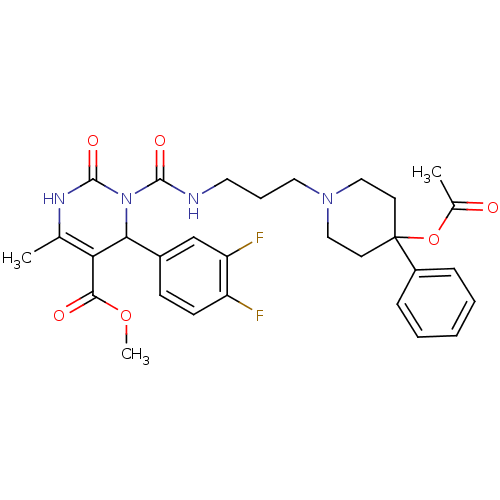 (3-[3-(4-Acetoxy-4-phenyl-piperidin-1-yl)-propylcar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50020310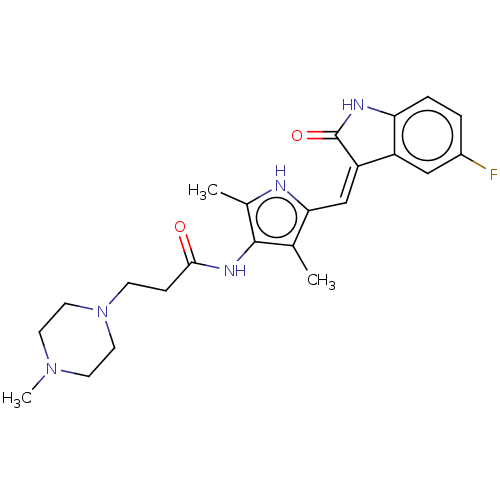 (CHEMBL3288854) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qilu Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Binding affinity to FLT3 (unknown origin) | Eur J Med Chem 82: 139-51 (2014) Article DOI: 10.1016/j.ejmech.2014.05.051 BindingDB Entry DOI: 10.7270/Q2F1918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 3 (Homo sapiens (Human)) | BDBM50020310 (CHEMBL3288854) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qilu Pharmaceutical Co, Ltd Curated by ChEMBL | Assay Description Binding affinity to VEGFR3 (unknown origin) | Eur J Med Chem 82: 139-51 (2014) Article DOI: 10.1016/j.ejmech.2014.05.051 BindingDB Entry DOI: 10.7270/Q2F1918D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1D adrenergic receptor of human liver microsomes. | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082823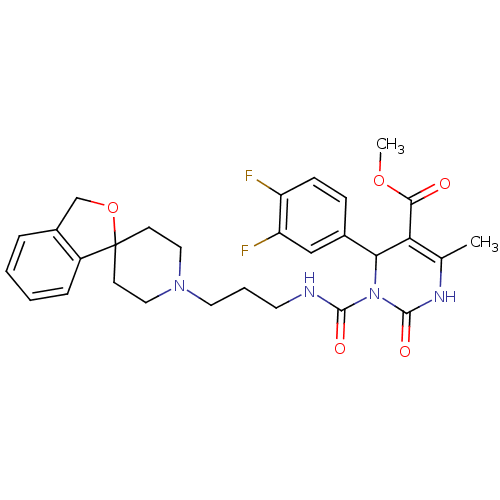 (CHEMBL145170 | methyl 4-(3,4-difluorophenyl)-6-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50090647 ((4S,5S)-4-(3,4-Difluoro-phenyl)-5-methyl-2-oxo-oxa...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity towards Alpha1A rat adrenergic receptors, using [125I]HEAT as radioligand. | J Med Chem 43: 2775-8 (2000) BindingDB Entry DOI: 10.7270/Q2PZ582K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50463294 (CHEMBL4249256) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 26: 4254-4263 (2018) Article DOI: 10.1016/j.bmc.2018.07.020 BindingDB Entry DOI: 10.7270/Q28W3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50463297 (CHEMBL4246433) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human MOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 26: 4254-4263 (2018) Article DOI: 10.1016/j.bmc.2018.07.020 BindingDB Entry DOI: 10.7270/Q28W3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082807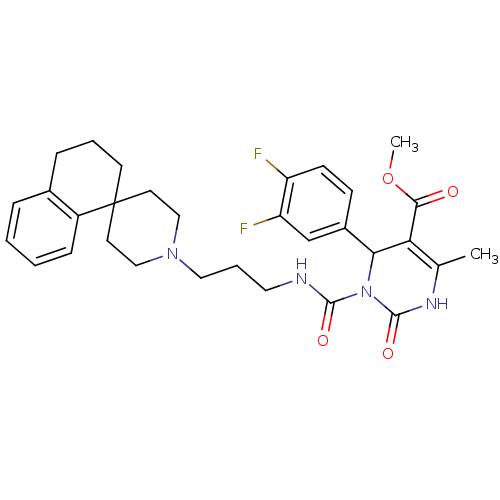 (CHEMBL344339 | methyl 4-(3,4-difluorophenyl)-6-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50506108 (CHEMBL4449252) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human KOR expressed in CHO cell membranes incubated for 30 mins by liquid scintillation counting | J Med Chem 62: 11054-11070 (2019) Article DOI: 10.1021/acs.jmedchem.9b00857 BindingDB Entry DOI: 10.7270/Q2VD72RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50244204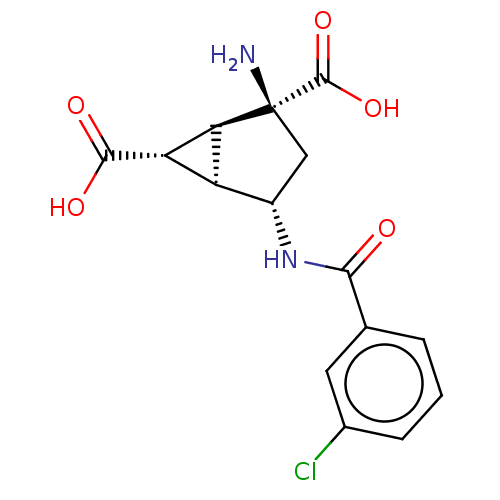 (CHEMBL4071962) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-LY459477 from recombinant human mGlu3 receptor expressed in HEK cell membranes after 90 mins by liquid scintillation counting | J Med Chem 61: 2303-2328 (2018) Article DOI: 10.1021/acs.jmedchem.7b01481 BindingDB Entry DOI: 10.7270/Q2W95CM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50506108 (CHEMBL4449252) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01082 BindingDB Entry DOI: 10.7270/Q2NZ8CG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082808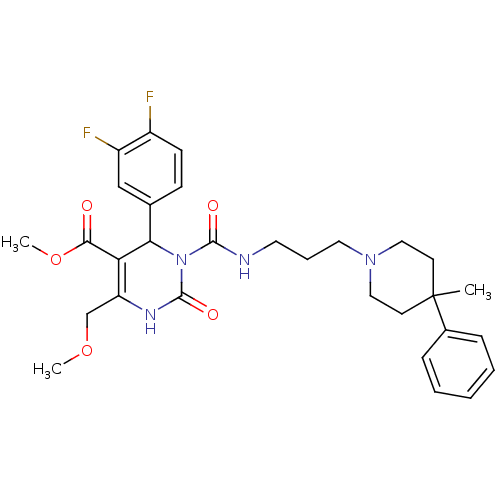 (4-(3,4-Difluoro-phenyl)-6-methoxymethyl-3-[3-(4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082833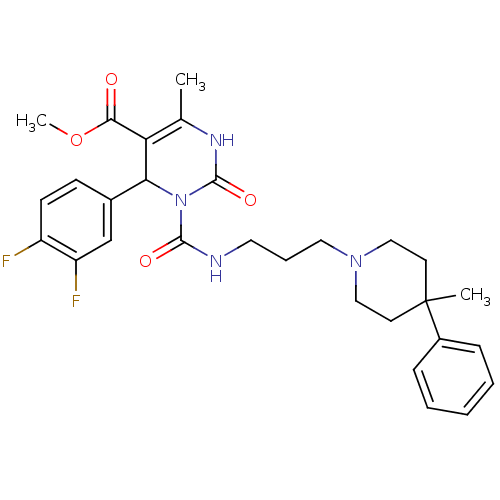 (4-(3,4-Difluoro-phenyl)-6-methyl-3-[3-(4-methyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50084999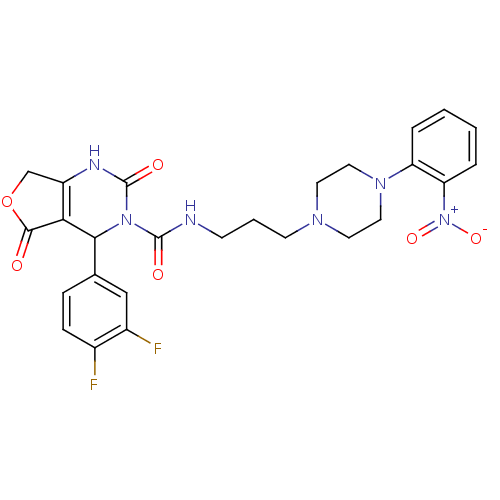 (4-(3,4-Difluoro-phenyl)-2,5-dioxo-1,2,5,7-tetrahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Displacement of [3H]- prazosin from human recombinant Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 10: 175-8 (2000) BindingDB Entry DOI: 10.7270/Q2N29W5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1B adrenergic receptor of human liver microsomes. | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50463297 (CHEMBL4246433) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 26: 4254-4263 (2018) Article DOI: 10.1016/j.bmc.2018.07.020 BindingDB Entry DOI: 10.7270/Q28W3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133054 (CHEMBL412228 | Opioid Peptide [d-Ala(8)]Dynorphin ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity of the compound towards rat Opioid receptor kappa 1 expressed in CHO cells of chinese hamster ovary was determined using [3H]-dipren... | J Med Chem 46: 4002-8 (2003) Article DOI: 10.1021/jm030075o BindingDB Entry DOI: 10.7270/Q2CN739H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133051 (CHEMBL413832 | Opioid Peptide [d-Ala(8)]Dynorphin ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity of the compound towards rat Opioid receptor kappa 1 expressed in CHO cells of chinese hamster ovary was determined using [3H]-dipren... | J Med Chem 46: 4002-8 (2003) Article DOI: 10.1021/jm030075o BindingDB Entry DOI: 10.7270/Q2CN739H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082815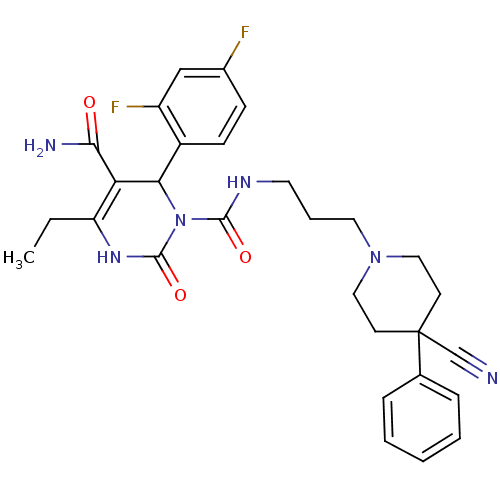 (6-(2,4-Difluoro-phenyl)-4-ethyl-2-oxo-3,6-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133050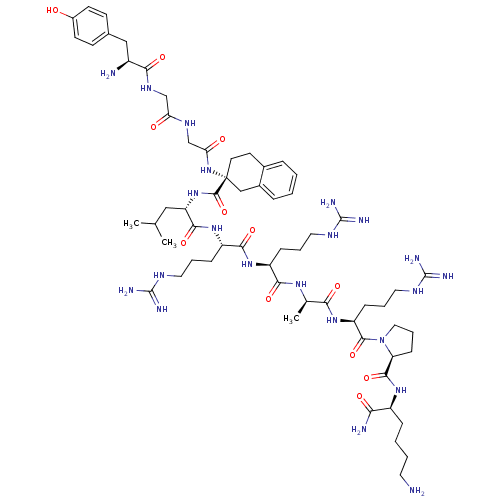 (CHEMBL385696 | Opioid Peptide [d-Ala(8)]Dynorphin ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity of the compound towards rat Opioid receptor kappa 1 expressed in CHO cells of chinese hamster ovary was determined using [3H]-dipren... | J Med Chem 46: 4002-8 (2003) Article DOI: 10.1021/jm030075o BindingDB Entry DOI: 10.7270/Q2CN739H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM85780 ([(R)-Atc4, D-Ala8]Dyn A-(1-11)NH2 | [(S)-Atc4 , D-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by PDSP Ki Database | Chirality 13: 125-9 (2001) Article DOI: 10.1002/1520-636X(2001)13:3 BindingDB Entry DOI: 10.7270/Q2SJ1J5H | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50085000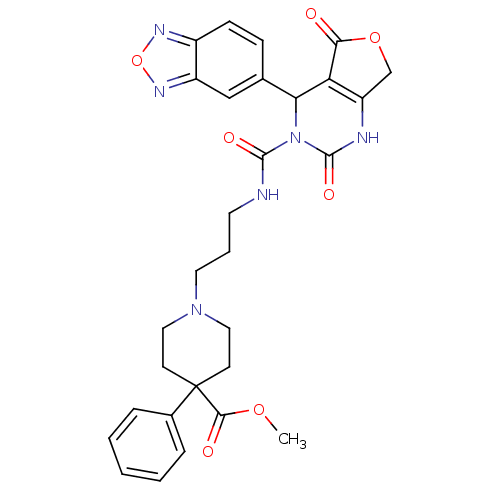 (1-{3-[(4-Benzo[1,2,5]oxadiazol-5-yl-2,5-dioxo-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Displacement of [3H]- prazosin from human recombinant Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 10: 175-8 (2000) BindingDB Entry DOI: 10.7270/Q2N29W5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50084996 (1-(3-{[4-(3,4-Difluoro-phenyl)-2,5-dioxo-1,2,5,7-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Displacement of [3H]- prazosin from human recombinant Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 10: 175-8 (2000) BindingDB Entry DOI: 10.7270/Q2N29W5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50244219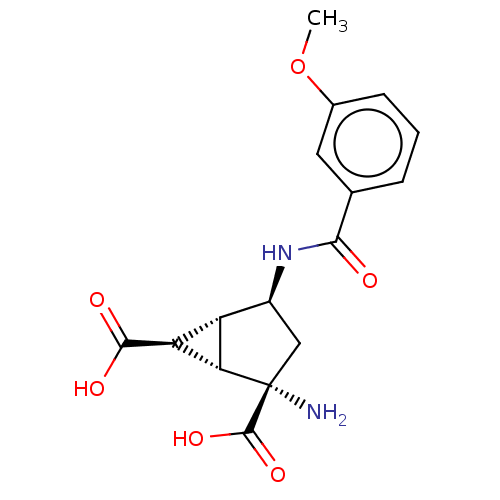 (CHEMBL4081453) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.927 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor from bovine hippocampus, used [3H]8-OH-DPAT as radioligand | J Med Chem 61: 2303-2328 (2018) Article DOI: 10.1021/acs.jmedchem.7b01481 BindingDB Entry DOI: 10.7270/Q2W95CM9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082829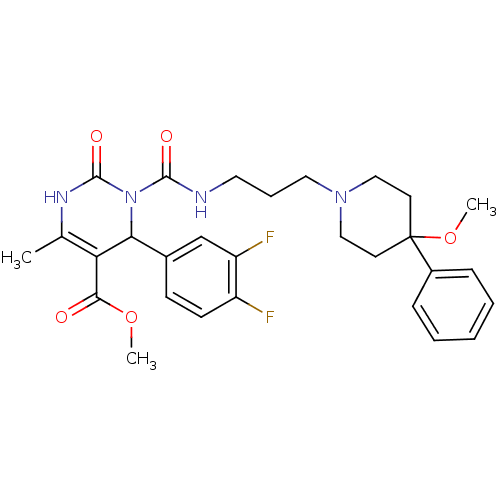 (4-(3,4-Difluoro-phenyl)-3-[3-(4-methoxy-4-phenyl-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082797 (4-(3,4-Difluoro-phenyl)-3-[3-(4-methoxycarbonyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Displacement of [3H]- prazosin from human recombinant Alpha-1D adrenergic receptor | Bioorg Med Chem Lett 10: 175-8 (2000) BindingDB Entry DOI: 10.7270/Q2N29W5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 8897 total ) | Next | Last >> |