| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Peroxisome proliferator-activated receptor alpha |
|---|
| Ligand | BDBM50540554 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1982232 (CHEMBL4615494) |
|---|
| IC50 | 330±n/a nM |
|---|
| Citation |  Ammazzalorso, A; Bruno, I; Florio, R; De Lellis, L; Laghezza, A; Cerchia, C; De Filippis, B; Fantacuzzi, M; Giampietro, L; Maccallini, C; Tortorella, P; Veschi, S; Loiodice, F; Lavecchia, A; Cama, A; Amoroso, R Sulfonimide and Amide Derivatives as Novel PPAR? Antagonists: Synthesis, Antiproliferative Activity, and Docking Studies. ACS Med Chem Lett11:624-632 (2020) [PubMed] Article Ammazzalorso, A; Bruno, I; Florio, R; De Lellis, L; Laghezza, A; Cerchia, C; De Filippis, B; Fantacuzzi, M; Giampietro, L; Maccallini, C; Tortorella, P; Veschi, S; Loiodice, F; Lavecchia, A; Cama, A; Amoroso, R Sulfonimide and Amide Derivatives as Novel PPAR? Antagonists: Synthesis, Antiproliferative Activity, and Docking Studies. ACS Med Chem Lett11:624-632 (2020) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Peroxisome proliferator-activated receptor alpha |
|---|
| Name: | Peroxisome proliferator-activated receptor alpha |
|---|
| Synonyms: | NR1C1 | Nuclear receptor subfamily 1 group C member 1 | PPAR | PPAR alpha/gamma | PPAR-alpha | PPARA | PPARA_HUMAN | Peroxisome Proliferator-Activated Receptor alpha | Peroxisome proliferator-activated receptor | Peroxisome proliferator-activated receptor alpha (PPAR alpha) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 52222.08 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q07869 |
|---|
| Residue: | 468 |
|---|
| Sequence: | MVDTESPLCPLSPLEAGDLESPLSEEFLQEMGNIQEISQSIGEDSSGSFGFTEYQYLGSC
PGSDGSVITDTLSPASSPSSVTYPVVPGSVDESPSGALNIECRICGDKASGYHYGVHACE
GCKGFFRRTIRLKLVYDKCDRSCKIQKKNRNKCQYCRFHKCLSVGMSHNAIRFGRMPRSE
KAKLKAEILTCEHDIEDSETADLKSLAKRIYEAYLKNFNMNKVKARVILSGKASNNPPFV
IHDMETLCMAEKTLVAKLVANGIQNKEAEVRIFHCCQCTSVETVTELTEFAKAIPGFANL
DLNDQVTLLKYGVYEAIFAMLSSVMNKDGMLVAYGNGFITREFLKSLRKPFCDIMEPKFD
FAMKFNALELDDSDISLFVAAIICCGDRPGLLNVGHIEKMQEGIVHVLRLHLQSNHPDDI
FLFPKLLQKMADLRQLVTEHAQLVQIIKKTESDAALHPLLQEIYRDMY
|
|
|
|---|
| BDBM50540554 |
|---|
| n/a |
|---|
| Name | BDBM50540554 |
|---|
| Synonyms: | CHEMBL4636810 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H31N3O6S |
|---|
| Mol. Mass. | 573.659 |
|---|
| SMILES | COc1ccc(cc1)S(=O)(=O)NC(=O)C(C)(C)Oc1ccc(CCOc2ccc(cc2)\N=N\c2ccccc2)cc1 |
|---|
| Structure | 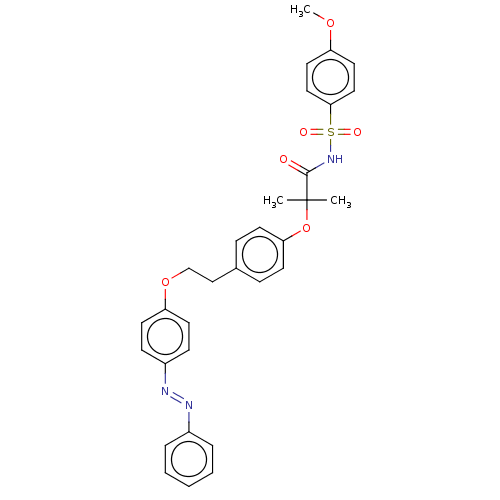
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ammazzalorso, A; Bruno, I; Florio, R; De Lellis, L; Laghezza, A; Cerchia, C; De Filippis, B; Fantacuzzi, M; Giampietro, L; Maccallini, C; Tortorella, P; Veschi, S; Loiodice, F; Lavecchia, A; Cama, A; Amoroso, R Sulfonimide and Amide Derivatives as Novel PPAR? Antagonists: Synthesis, Antiproliferative Activity, and Docking Studies. ACS Med Chem Lett11:624-632 (2020) [PubMed] Article
Ammazzalorso, A; Bruno, I; Florio, R; De Lellis, L; Laghezza, A; Cerchia, C; De Filippis, B; Fantacuzzi, M; Giampietro, L; Maccallini, C; Tortorella, P; Veschi, S; Loiodice, F; Lavecchia, A; Cama, A; Amoroso, R Sulfonimide and Amide Derivatives as Novel PPAR? Antagonists: Synthesis, Antiproliferative Activity, and Docking Studies. ACS Med Chem Lett11:624-632 (2020) [PubMed] Article