| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2D6 |
|---|
| Ligand | BDBM50338821 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1295001 (CHEMBL3129312) |
|---|
| IC50 | 2200±n/a nM |
|---|
| Citation |  Moyes, CR; Berger, R; Goble, SD; Harper, B; Shen, DM; Wang, L; Bansal, A; Brown, PN; Chen, AS; Dingley, KH; Di Salvo, J; Fitzmaurice, A; Gichuru, LN; Hurley, AL; Jochnowitz, N; Miller, RR; Mistry, S; Nagabukuro, H; Salituro, GM; Sanfiz, A; Stevenson, AS; Villa, K; Zamlynny, B; Struthers, M; Weber, AE; Edmondson, SD Design, synthesis, and evaluation of conformationally restricted acetanilides as potent and selective▀3 adrenergic receptor agonists for the treatment of overactive bladder. J Med Chem57:1437-53 (2014) [PubMed] Article Moyes, CR; Berger, R; Goble, SD; Harper, B; Shen, DM; Wang, L; Bansal, A; Brown, PN; Chen, AS; Dingley, KH; Di Salvo, J; Fitzmaurice, A; Gichuru, LN; Hurley, AL; Jochnowitz, N; Miller, RR; Mistry, S; Nagabukuro, H; Salituro, GM; Sanfiz, A; Stevenson, AS; Villa, K; Zamlynny, B; Struthers, M; Weber, AE; Edmondson, SD Design, synthesis, and evaluation of conformationally restricted acetanilides as potent and selective▀3 adrenergic receptor agonists for the treatment of overactive bladder. J Med Chem57:1437-53 (2014) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2D6 |
|---|
| Name: | Cytochrome P450 2D6 |
|---|
| Synonyms: | CP2D6_HUMAN | CYP2D6 | CYP2DL1 | CYPIID6 | Cytochrome P450 2D6 (CYP2D6) | Debrisoquine 4-hydroxylase | P450-DB1 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 55774.82 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P10635 |
|---|
| Residue: | 497 |
|---|
| Sequence: | MGLEALVPLAVIVAIFLLLVDLMHRRQRWAARYPPGPLPLPGLGNLLHVDFQNTPYCFDQ
LRRRFGDVFSLQLAWTPVVVLNGLAAVREALVTHGEDTADRPPVPITQILGFGPRSQGVF
LARYGPAWREQRRFSVSTLRNLGLGKKSLEQWVTEEAACLCAAFANHSGRPFRPNGLLDK
AVSNVIASLTCGRRFEYDDPRFLRLLDLAQEGLKEESGFLREVLNAVPVLLHIPALAGKV
LRFQKAFLTQLDELLTEHRMTWDPAQPPRDLTEAFLAEMEKAKGNPESSFNDENLRIVVA
DLFSAGMVTTSTTLAWGLLLMILHPDVQRRVQQEIDDVIGQVRRPEMGDQAHMPYTTAVI
HEVQRFGDIVPLGVTHMTSRDIEVQGFRIPKGTTLITNLSSVLKDEAVWEKPFRFHPEHF
LDAQGHFVKPEAFLPFSAGRRACLGEPLARMELFLFFTSLLQHFSFSVPTGQPRPSHHGV
FAFLVSPSPYELCAVPR
|
|
|
|---|
| BDBM50338821 |
|---|
| n/a |
|---|
| Name | BDBM50338821 |
|---|
| Synonyms: | 2-(2-aminothiazol-4-yl)-N-(4-(((2S,5R)-5-((R)-hydroxy(phenyl)methyl)pyrrolidin-2-yl)methyl)phenyl)acetamide | CHEMBL1684585 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H26N4O2S |
|---|
| Mol. Mass. | 422.543 |
|---|
| SMILES | Nc1nc(CC(=O)Nc2ccc(C[C@@H]3CC[C@@H](N3)[C@H](O)c3ccccc3)cc2)cs1 |r| |
|---|
| Structure | 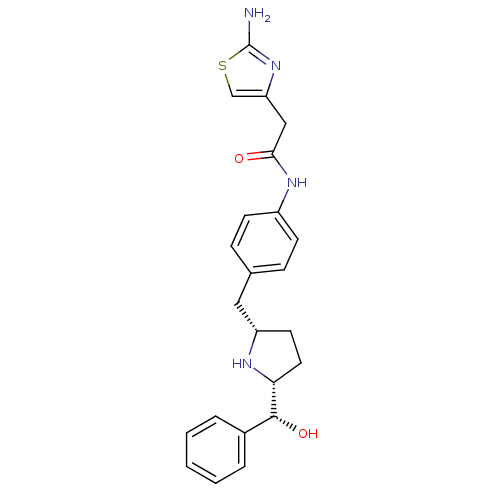
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Moyes, CR; Berger, R; Goble, SD; Harper, B; Shen, DM; Wang, L; Bansal, A; Brown, PN; Chen, AS; Dingley, KH; Di Salvo, J; Fitzmaurice, A; Gichuru, LN; Hurley, AL; Jochnowitz, N; Miller, RR; Mistry, S; Nagabukuro, H; Salituro, GM; Sanfiz, A; Stevenson, AS; Villa, K; Zamlynny, B; Struthers, M; Weber, AE; Edmondson, SD Design, synthesis, and evaluation of conformationally restricted acetanilides as potent and selective▀3 adrenergic receptor agonists for the treatment of overactive bladder. J Med Chem57:1437-53 (2014) [PubMed] Article
Moyes, CR; Berger, R; Goble, SD; Harper, B; Shen, DM; Wang, L; Bansal, A; Brown, PN; Chen, AS; Dingley, KH; Di Salvo, J; Fitzmaurice, A; Gichuru, LN; Hurley, AL; Jochnowitz, N; Miller, RR; Mistry, S; Nagabukuro, H; Salituro, GM; Sanfiz, A; Stevenson, AS; Villa, K; Zamlynny, B; Struthers, M; Weber, AE; Edmondson, SD Design, synthesis, and evaluation of conformationally restricted acetanilides as potent and selective▀3 adrenergic receptor agonists for the treatment of overactive bladder. J Med Chem57:1437-53 (2014) [PubMed] Article