

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468144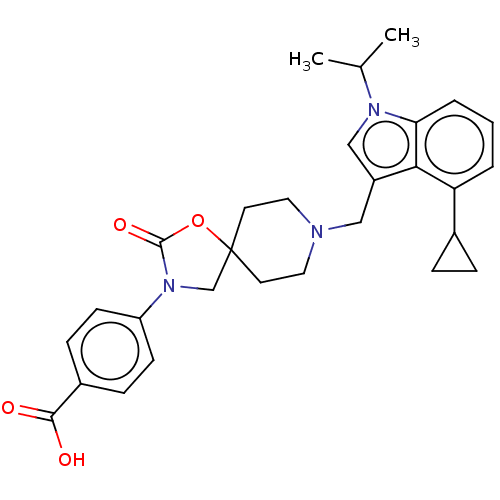 (CHEMBL4286244) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468136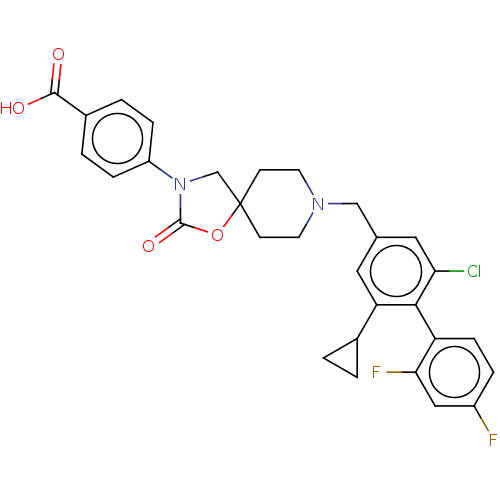 (CHEMBL4287248) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468160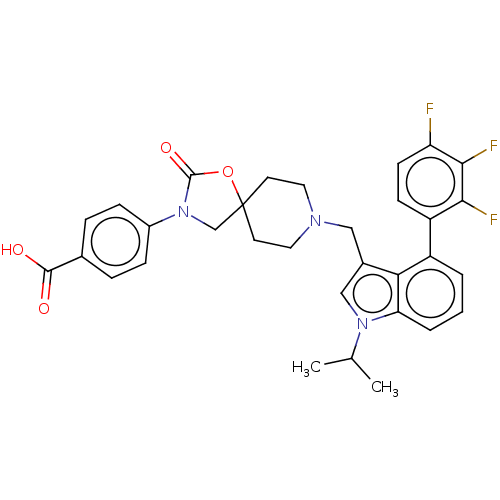 (CHEMBL4293458) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468144 (CHEMBL4286244) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Mus musculus) | BDBM123216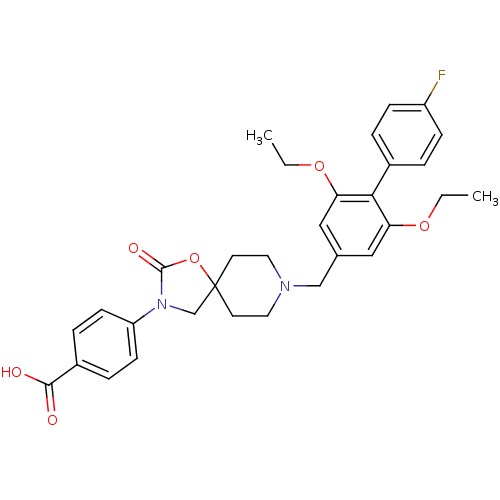 (US8742110, 3-1) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468157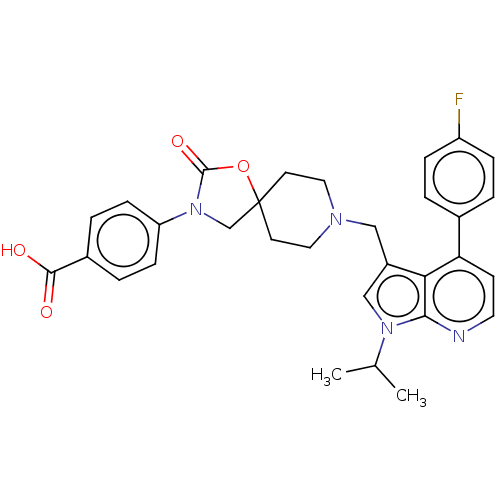 (CHEMBL4289661) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468136 (CHEMBL4287248) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468146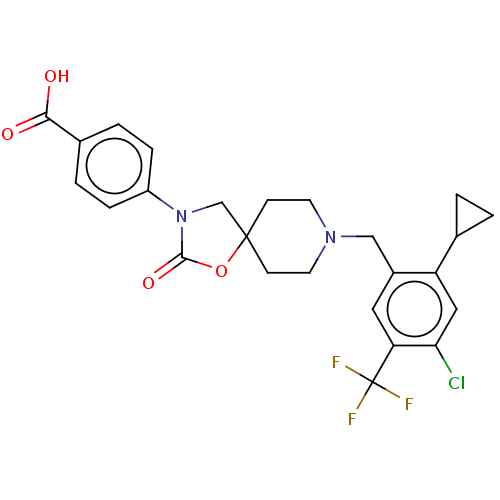 (CHEMBL4285917) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468157 (CHEMBL4289661) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468160 (CHEMBL4293458) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468153 (CHEMBL4279392) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468146 (CHEMBL4285917) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468159 (CHEMBL4278328) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468150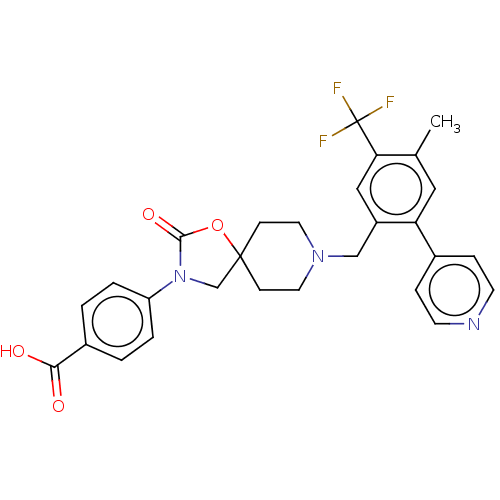 (CHEMBL4277006) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123216 (US8742110, 3-1) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468159 (CHEMBL4278328) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Mus musculus) | BDBM50468139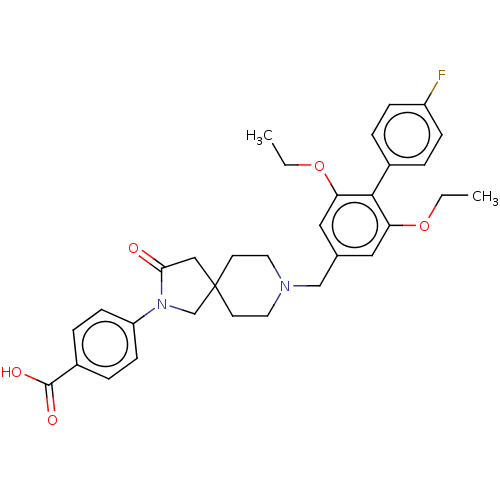 (CHEMBL4288391) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468145 (CHEMBL4278915) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Mus musculus) | BDBM50468160 (CHEMBL4293458) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123274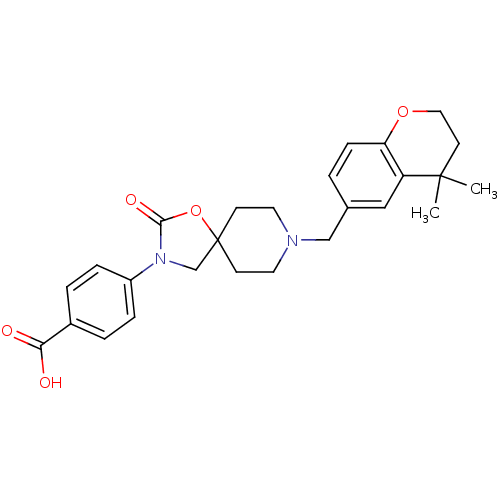 (US8742110, 5-10) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468139 (CHEMBL4288391) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468139 (CHEMBL4288391) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468154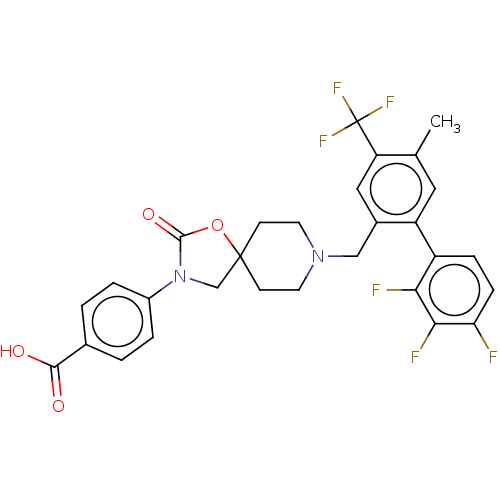 (CHEMBL4284956) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468143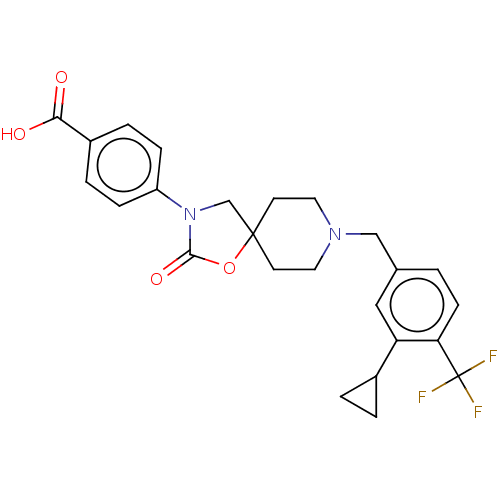 (CHEMBL4281446) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123274 (US8742110, 5-10) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123216 (US8742110, 3-1) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468153 (CHEMBL4279392) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Mus musculus) | BDBM50468136 (CHEMBL4287248) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468143 (CHEMBL4281446) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468148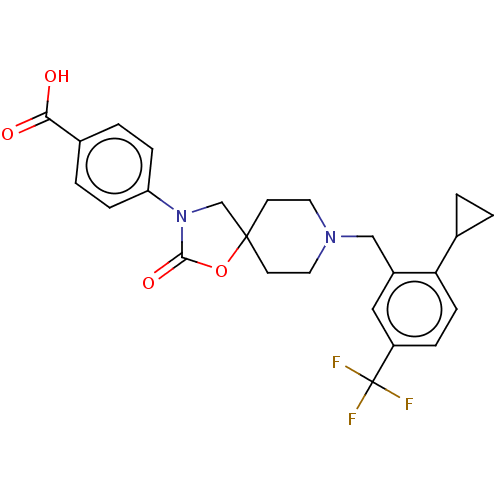 (CHEMBL4289325) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468148 (CHEMBL4289325) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468140 (CHEMBL4288334) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468150 (CHEMBL4277006) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468140 (CHEMBL4288334) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468161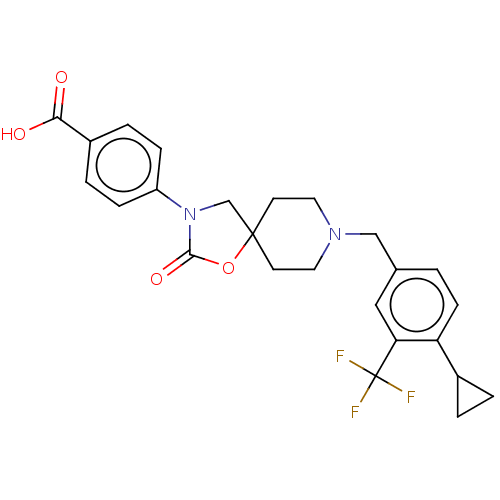 (CHEMBL4277991) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468154 (CHEMBL4284956) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468161 (CHEMBL4277991) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468142 (CHEMBL4280432) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468145 (CHEMBL4278915) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468155 (CHEMBL4277613) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Mus musculus) | BDBM50468144 (CHEMBL4286244) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468138 (CHEMBL4292738) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468155 (CHEMBL4277613) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468138 (CHEMBL4292738) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Mus musculus) | BDBM50468143 (CHEMBL4281446) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Mus musculus) | BDBM50468157 (CHEMBL4289661) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468152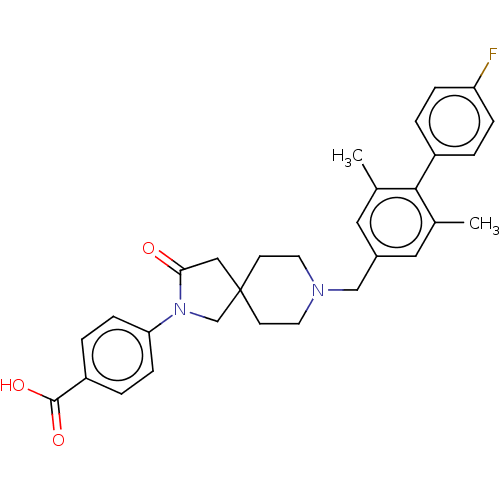 (CHEMBL4295189) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Mus musculus) | BDBM50468141 (CHEMBL4282854) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468137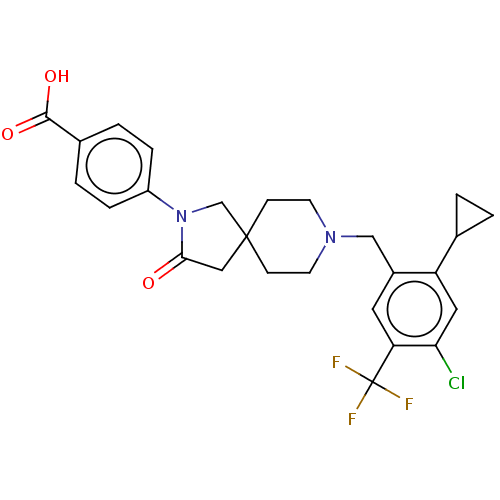 (CHEMBL4288259) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468142 (CHEMBL4280432) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 305 total ) | Next | Last >> |