| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Dipeptidyl peptidase 8 |
|---|
| Ligand | BDBM12656 |
|---|
| Substrate/Competitor | BDBM11057 |
|---|
| Meas. Tech. | DPP Inhibition Assay |
|---|
| pH | 7.5±n/a |
|---|
| Temperature | 295.15±n/a K |
|---|
| Ki | >30000±n/a nM |
|---|
| Citation |  Pei, Z; Li, X; von Geldern, TW; Madar, DJ; Longenecker, K; Yong, H; Lubben, TH; Stewart, KD; Zinker, BA; Backes, BJ; Judd, AS; Mulhern, M; Ballaron, SJ; Stashko, MA; Mika, AK; Beno, DW; Reinhart, GA; Fryer, RM; Preusser, LC; Kempf-Grote, AJ; Sham, HL; Trevillyan, JM Discovery of ((4R,5S)-5-amino-4-(2,4,5- trifluorophenyl)cyclohex-1-enyl)-(3- (trifluoromethyl)-5,6-dihydro- [1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)methanone (ABT-341), a highly potent, selective, orally efficacious, and safe dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem49:6439-42 (2006) [PubMed] Article Pei, Z; Li, X; von Geldern, TW; Madar, DJ; Longenecker, K; Yong, H; Lubben, TH; Stewart, KD; Zinker, BA; Backes, BJ; Judd, AS; Mulhern, M; Ballaron, SJ; Stashko, MA; Mika, AK; Beno, DW; Reinhart, GA; Fryer, RM; Preusser, LC; Kempf-Grote, AJ; Sham, HL; Trevillyan, JM Discovery of ((4R,5S)-5-amino-4-(2,4,5- trifluorophenyl)cyclohex-1-enyl)-(3- (trifluoromethyl)-5,6-dihydro- [1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)methanone (ABT-341), a highly potent, selective, orally efficacious, and safe dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem49:6439-42 (2006) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Solution Info, Assay Method |
|---|
| |
| Dipeptidyl peptidase 8 |
|---|
| Name: | Dipeptidyl peptidase 8 |
|---|
| Synonyms: | DPP8 | DPP8_HUMAN | DPRP-1 | DPRP1 | Dipeptidyl peptidase 8 (DPP-8) | Dipeptidyl peptidase 8 (DPP8) | Dipeptidyl peptidase 8/9 | Dipeptidyl peptidase IV-related protein 1 | Dipeptidyl peptidase VIII | Dipeptidyl peptidase VIII (DDP-VIII) | Prolyl dipeptidase DPP8 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 103342.62 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q6V1X1 |
|---|
| Residue: | 898 |
|---|
| Sequence: | MWKRSEQMKIKSGKCNMAAAMETEQLGVEIFETADCEENIESQDRPKLEPFYVERYSWSQ
LKKLLADTRKYHGYMMAKAPHDFMFVKRNDPDGPHSDRIYYLAMSGENRENTLFYSEIPK
TINRAAVLMLSWKPLLDLFQATLDYGMYSREEELLRERKRIGTVGIASYDYHQGSGTFLF
QAGSGIYHVKDGGPQGFTQQPLRPNLVETSCPNIRMDPKLCPADPDWIAFIHSNDIWISN
IVTREERRLTYVHNELANMEEDARSAGVATFVLQEEFDRYSGYWWCPKAETTPSGGKILR
ILYEENDESEVEIIHVTSPMLETRRADSFRYPKTGTANPKVTFKMSEIMIDAEGRIIDVI
DKELIQPFEILFEGVEYIARAGWTPEGKYAWSILLDRSQTRLQIVLISPELFIPVEDDVM
ERQRLIESVPDSVTPLIIYEETTDIWINIHDIFHVFPQSHEEEIEFIFASECKTGFRHLY
KITSILKESKYKRSSGGLPAPSDFKCPIKEEIAITSGEWEVLGRHGSNIQVDEVRRLVYF
EGTKDSPLEHHLYVVSYVNPGEVTRLTDRGYSHSCCISQHCDFFISKYSNQKNPHCVSLY
KLSSPEDDPTCKTKEFWATILDSAGPLPDYTPPEIFSFESTTGFTLYGMLYKPHDLQPGK
KYPTVLFIYGGPQVQLVNNRFKGVKYFRLNTLASLGYVVVVIDNRGSCHRGLKFEGAFKY
KMGQIEIDDQVEGLQYLASRYDFIDLDRVGIHGWSYGGYLSLMALMQRSDIFRVAIAGAP
VTLWIFYDTGYTERYMGHPDQNEQGYYLGSVAMQAEKFPSEPNRLLLLHGFLDENVHFAH
TSILLSFLVRAGKPYDLQIYPQERHSIRVPESGEHYELHLLHYLQENLGSRIAALKVI
|
|
|
|---|
| BDBM12656 |
|---|
| BDBM11057 |
|---|
| Name | BDBM12656 |
|---|
| Synonyms: | ((4R,5S)-5-Amino-4-(2,4,5-trifluorophenyl)cyclohex-1-enyl)-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)methanone | (1S,6R)-3-{[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]carbonyl}-6-(2,4,5-trifluorophenyl)cyclohex-3-en-1-aminium trifluoroacetate | (1S,6R)-3-{[3-(trifluoromethyl)-5H,6H,7H,8H-[1,2,4]triazolo[3,4-a]pyrazin-7-yl]carbonyl}-6-(2,4,5-trifluorophenyl)cyclohex-3-en-1-aminium; 2,2,2-trifluoroacetate | ABT-341 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H18F6N5O |
|---|
| Mol. Mass. | 446.369 |
|---|
| SMILES | [NH3+][C@H]1CC(=CC[C@@H]1c1cc(F)c(F)cc1F)C(=O)N1CCn2c(C1)nnc2C(F)(F)F |r,c:3| |
|---|
| Structure | 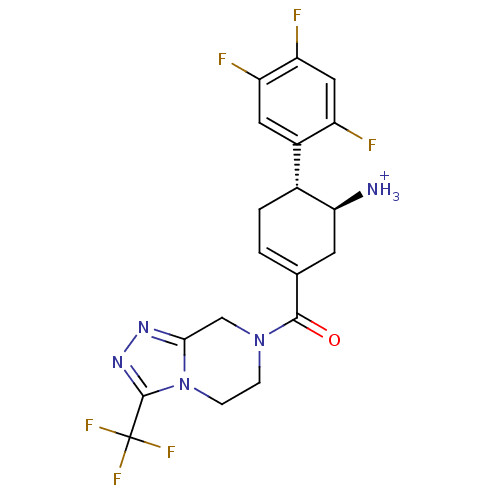
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Pei, Z; Li, X; von Geldern, TW; Madar, DJ; Longenecker, K; Yong, H; Lubben, TH; Stewart, KD; Zinker, BA; Backes, BJ; Judd, AS; Mulhern, M; Ballaron, SJ; Stashko, MA; Mika, AK; Beno, DW; Reinhart, GA; Fryer, RM; Preusser, LC; Kempf-Grote, AJ; Sham, HL; Trevillyan, JM Discovery of ((4R,5S)-5-amino-4-(2,4,5- trifluorophenyl)cyclohex-1-enyl)-(3- (trifluoromethyl)-5,6-dihydro- [1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)methanone (ABT-341), a highly potent, selective, orally efficacious, and safe dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem49:6439-42 (2006) [PubMed] Article
Pei, Z; Li, X; von Geldern, TW; Madar, DJ; Longenecker, K; Yong, H; Lubben, TH; Stewart, KD; Zinker, BA; Backes, BJ; Judd, AS; Mulhern, M; Ballaron, SJ; Stashko, MA; Mika, AK; Beno, DW; Reinhart, GA; Fryer, RM; Preusser, LC; Kempf-Grote, AJ; Sham, HL; Trevillyan, JM Discovery of ((4R,5S)-5-amino-4-(2,4,5- trifluorophenyl)cyclohex-1-enyl)-(3- (trifluoromethyl)-5,6-dihydro- [1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)methanone (ABT-341), a highly potent, selective, orally efficacious, and safe dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem49:6439-42 (2006) [PubMed] Article