

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224191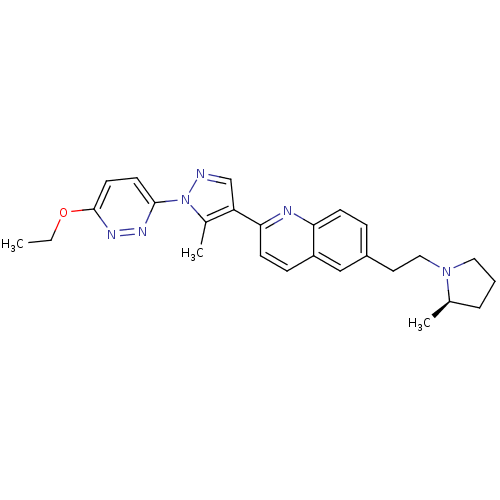 ((R)-2-(1-(6-ethoxypyridazin-3-yl)-5-methyl-1H-pyra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224192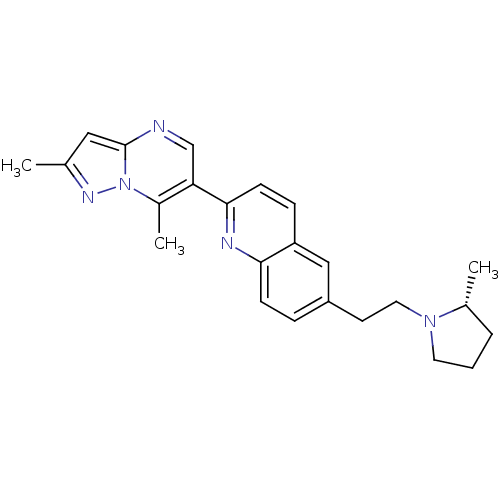 ((R)-2-(2,7-dimethylpyrazolo[1,5-a]pyrimidin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224187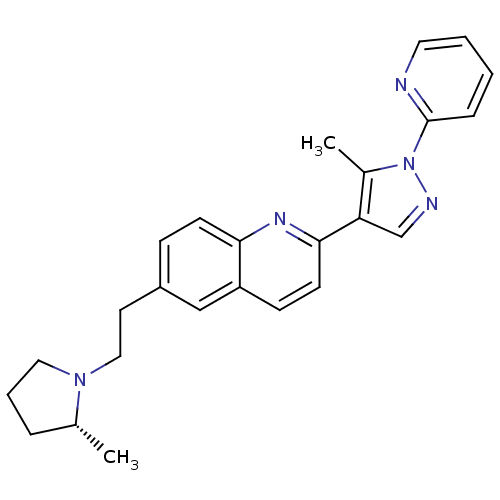 ((R)-2-(5-methyl-1-(pyridin-2-yl)-1H-pyrazol-4-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224189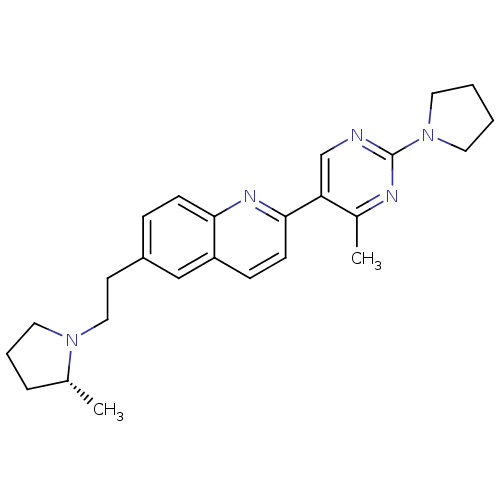 ((R)-2-(4-methyl-2-(pyrrolidin-1-yl)pyrimidin-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224188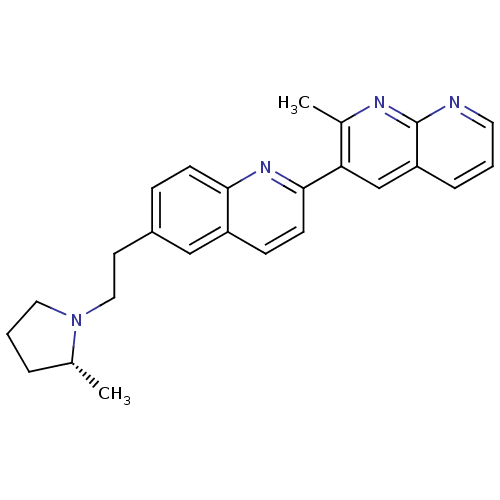 ((R)-2-methyl-3-(6-(2-(2-methylpyrrolidin-1-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224190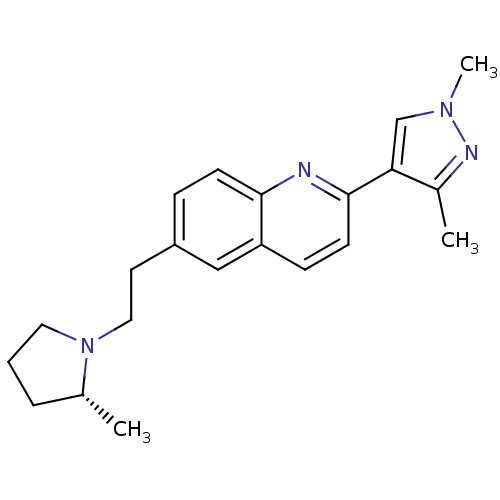 ((R)-2-(1,3-dimethyl-1H-pyrazol-4-yl)-6-(2-(2-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224191 ((R)-2-(1-(6-ethoxypyridazin-3-yl)-5-methyl-1H-pyra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from rat H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224189 ((R)-2-(4-methyl-2-(pyrrolidin-1-yl)pyrimidin-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from rat H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224192 ((R)-2-(2,7-dimethylpyrazolo[1,5-a]pyrimidin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as inhibition of RAMH-stimulated [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224187 ((R)-2-(5-methyl-1-(pyridin-2-yl)-1H-pyrazol-4-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from rat H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224192 ((R)-2-(2,7-dimethylpyrazolo[1,5-a]pyrimidin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from rat H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224191 ((R)-2-(1-(6-ethoxypyridazin-3-yl)-5-methyl-1H-pyra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as inhibition of RAMH-stimulated [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11644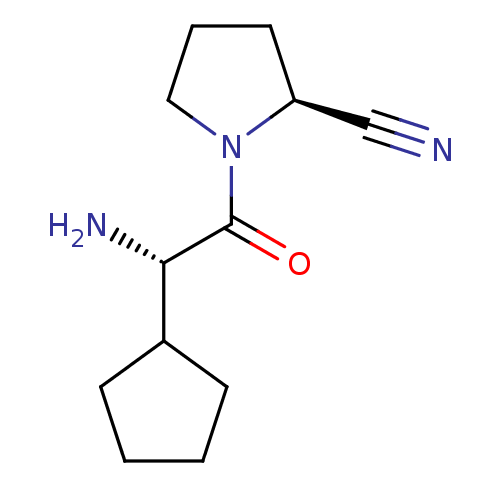 ((2S)-1-[(2S)-2-amino-2-cyclopentylacetyl]pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12648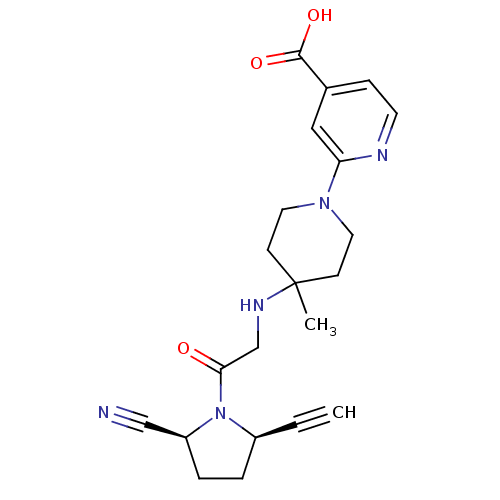 ((2S,5R)-5-Ethynyl-1-{N-(4-methyl-1-(4-carboxy-pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12656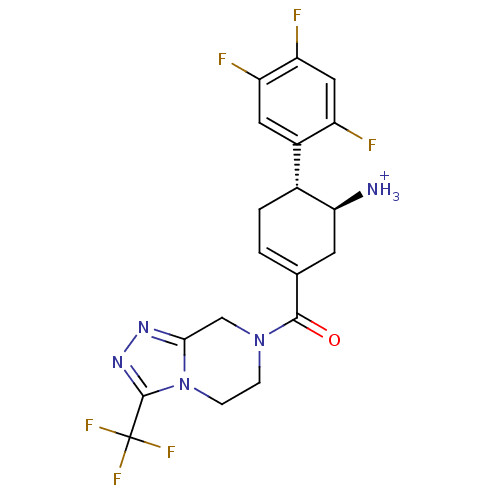 (((4R,5S)-5-Amino-4-(2,4,5-trifluorophenyl)cyclohex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | -50.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | J Med Chem 49: 6439-42 (2006) Article DOI: 10.1021/jm060955d BindingDB Entry DOI: 10.7270/Q2G73BXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12647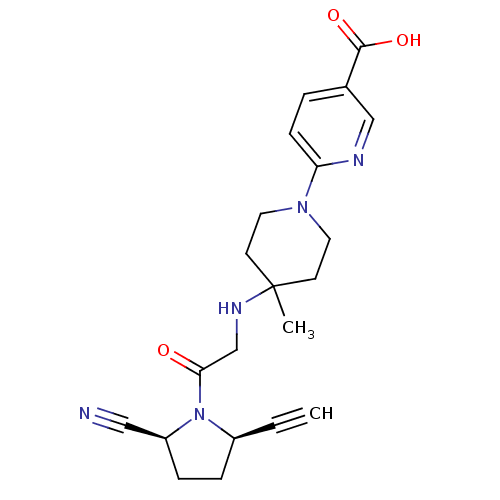 ((2S,5R)-5-ethynyl-1-(N-(4-methyl-1-(5-carboxy-pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from rat H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224190 ((R)-2-(1,3-dimethyl-1H-pyrazol-4-yl)-6-(2-(2-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from rat H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224188 ((R)-2-methyl-3-(6-(2-(2-methylpyrrolidin-1-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from rat H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224189 ((R)-2-(4-methyl-2-(pyrrolidin-1-yl)pyrimidin-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as inhibition of RAMH-stimulated [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12646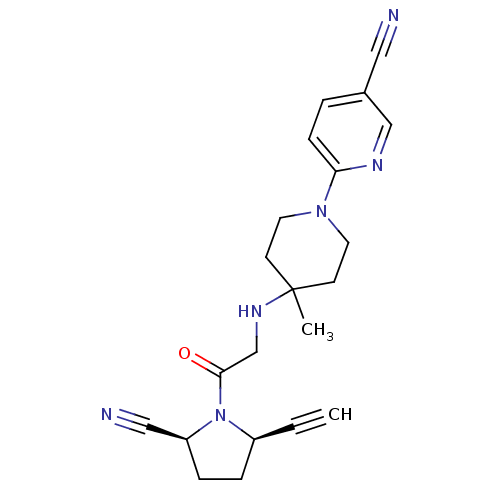 ((2S,5R)-5-ethynyl-1-(N-(4-methyl-1-(5-cyano-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM11644 ((2S)-1-[(2S)-2-amino-2-cyclopentylacetyl]pyrrolidi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224190 ((R)-2-(1,3-dimethyl-1H-pyrazol-4-yl)-6-(2-(2-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as inhibition of RAMH-stimulated [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224188 ((R)-2-methyl-3-(6-(2-(2-methylpyrrolidin-1-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as inhibition of RAMH-stimulated [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12654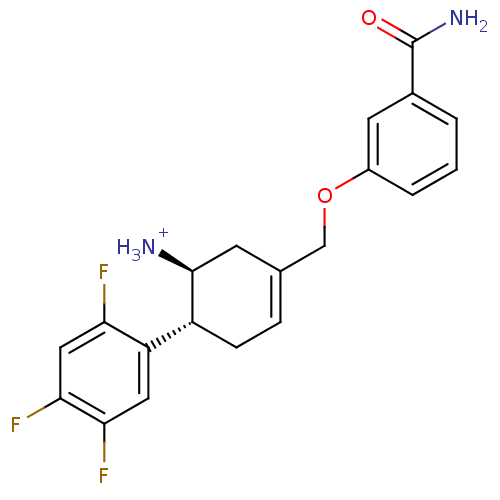 ((1S,6R)-3-(3-carbamoylphenoxymethyl)-6-(2,4,5-trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60 | -47.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | J Med Chem 49: 6439-42 (2006) Article DOI: 10.1021/jm060955d BindingDB Entry DOI: 10.7270/Q2G73BXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12643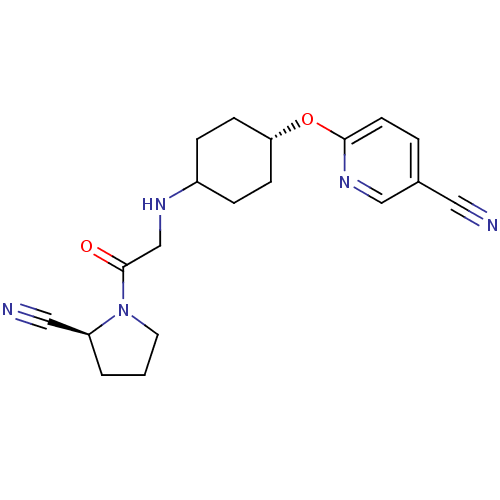 (6-{[4-({2-[(2S)-2-cyanopyrrolidin-1-yl]-2-oxoethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11644 ((2S)-1-[(2S)-2-amino-2-cyclopentylacetyl]pyrrolidi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as inhibition of RAMH-stimulated [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12655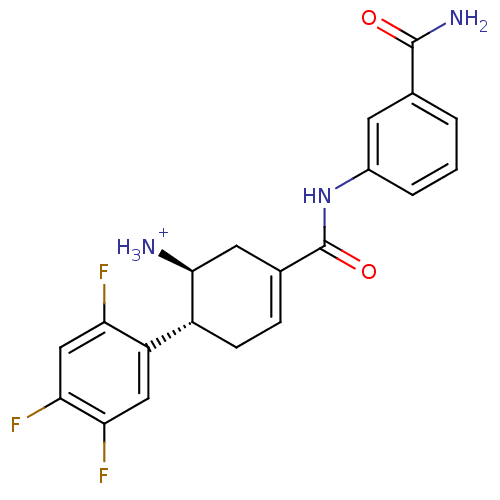 ((1S,6R)-3-[(3-carbamoylphenyl)carbamoyl]-6-(2,4,5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.70 | -47.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | J Med Chem 49: 6439-42 (2006) Article DOI: 10.1021/jm060955d BindingDB Entry DOI: 10.7270/Q2G73BXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12638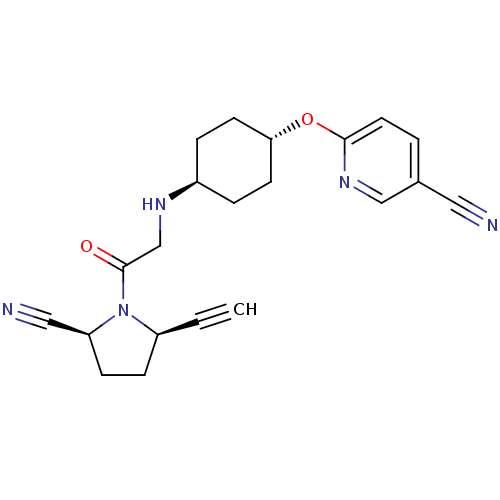 ((2S,5R)-5-ethynyl-1-{N-(4-trans(5-cyano-pyridin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12653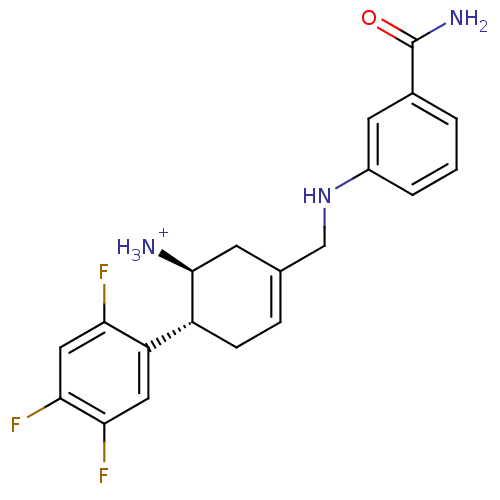 ((1S,6R)-3-{[(3-carbamoylphenyl)amino]methyl}-6-(2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | J Med Chem 49: 6439-42 (2006) Article DOI: 10.1021/jm060955d BindingDB Entry DOI: 10.7270/Q2G73BXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50224187 ((R)-2-(5-methyl-1-(pyridin-2-yl)-1H-pyrazol-4-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in HEK239 cells assessed as inhibition of RAMH-stimulated [35S]GTPgammaS binding | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12626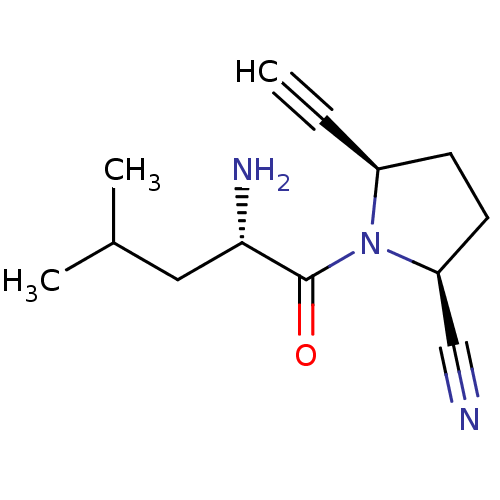 ((2S,5R)-1-[(2S)-2-amino-4-methylpentanoyl]-5-ethyn...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11642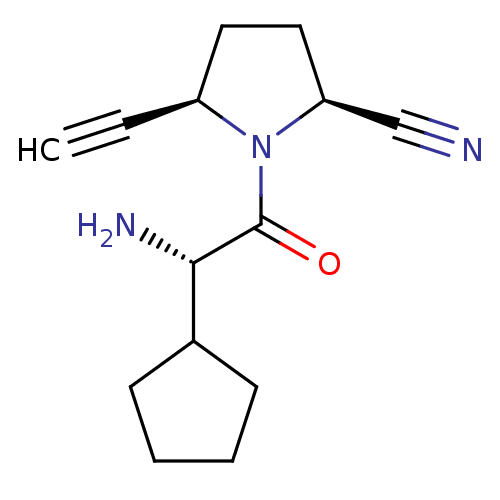 ((2S,5R)-1-[(2S)-2-amino-2-cyclopentylacetyl]-5-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12644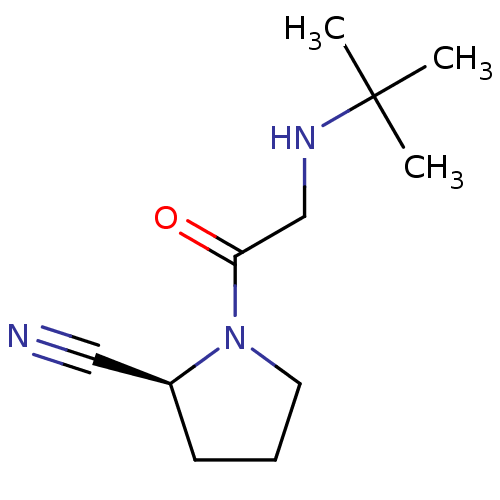 ((2S)-1-[2-(tert-butylamino)acetyl]pyrrolidine-2-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM12643 (6-{[4-({2-[(2S)-2-cyanopyrrolidin-1-yl]-2-oxoethyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12645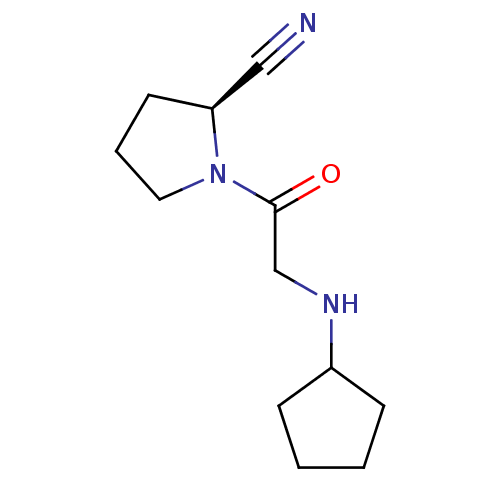 ((2S)-1-[2-(cyclopentylamino)acetyl]pyrrolidine-2-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12652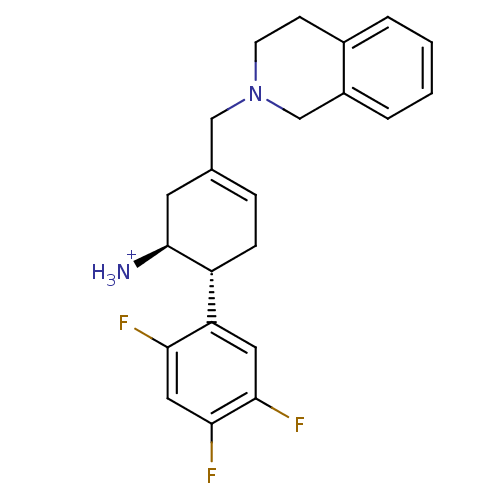 ((1S,6R)-3-(1,2,3,4-tetrahydroisoquinolin-2-ylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | -44.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | J Med Chem 49: 6439-42 (2006) Article DOI: 10.1021/jm060955d BindingDB Entry DOI: 10.7270/Q2G73BXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12635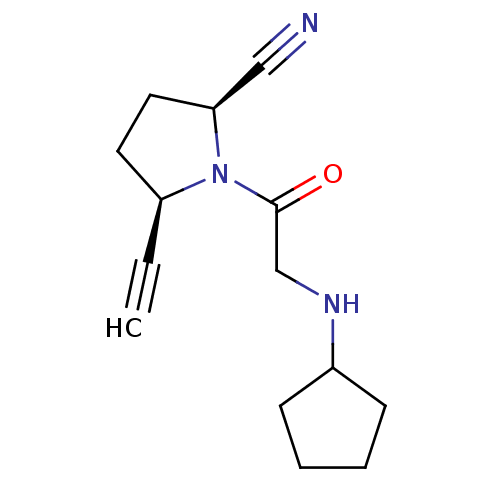 ((2S,5R)-1-(N-cyclopentylglycyl)-5-ethynylpyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 22 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11643 ((2S,5R)-1-((2S)-2-amino-2-cyclopentylethanoyl)-5-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 24 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12639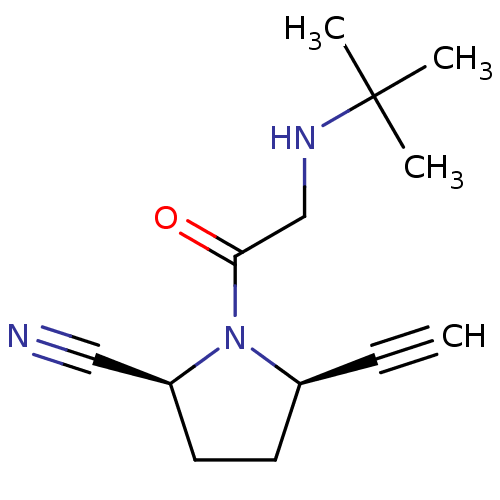 ((2S,5R)-1-(N-(tert-butyl)glycyl)-5-ethynylpyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12651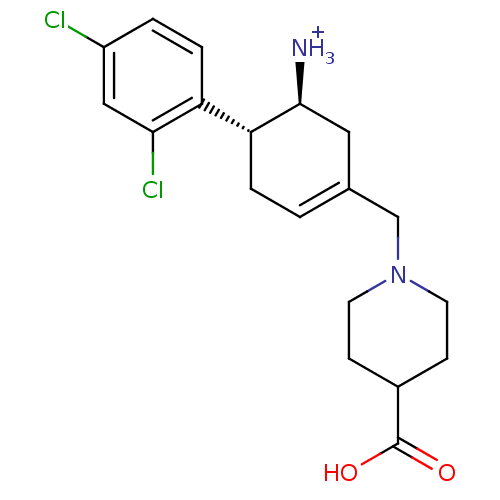 ((1S,6R)-3-[(4-carboxypiperidin-1-yl)methyl]-6-(2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 45 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | J Med Chem 49: 6439-42 (2006) Article DOI: 10.1021/jm060955d BindingDB Entry DOI: 10.7270/Q2G73BXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12627 ((2S,5S)-1-L-leucyl-5-methylpyrrolidine-2-carbonitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 49 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12642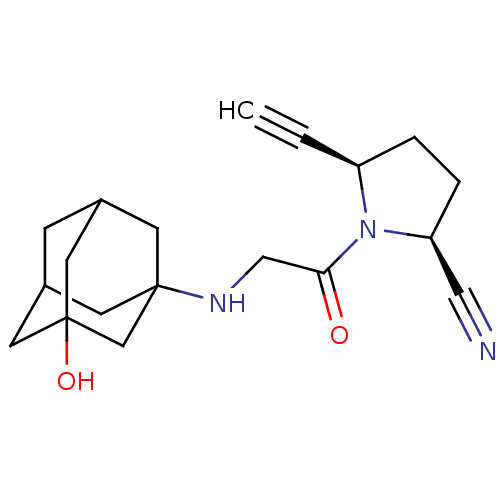 ((2S,5R)-5-ethynyl-1-[N-(3-hydroxy-1-adamantyl)glyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM12652 ((1S,6R)-3-(1,2,3,4-tetrahydroisoquinolin-2-ylmethy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 73 | -40.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... | J Med Chem 49: 6439-42 (2006) Article DOI: 10.1021/jm060955d BindingDB Entry DOI: 10.7270/Q2G73BXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12636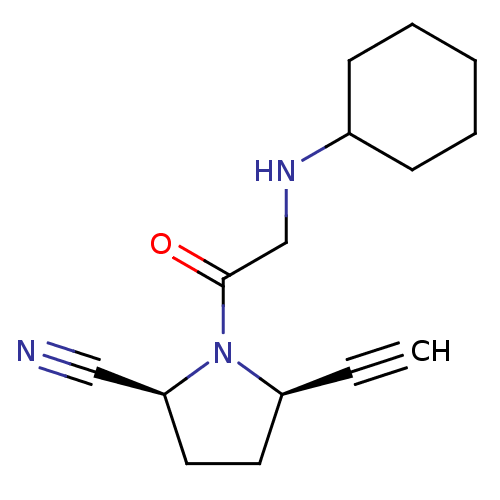 ((2S,5R)-1-(N-cyclohexylglycyl)-5-ethynylpyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 79 | -40.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12637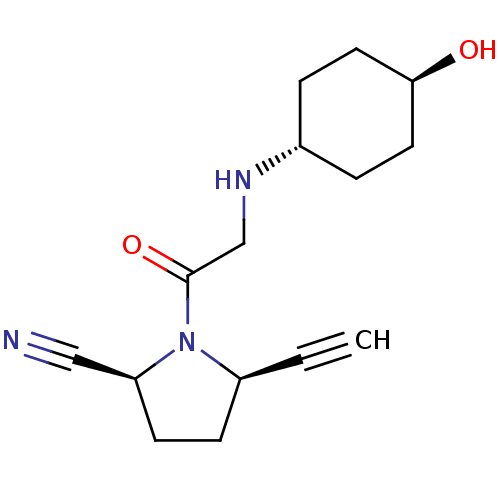 ((2S,5R)-5-ethynyl-1-(N-trans(4-hydroxycyclohexyl)g...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 566 total ) | Next | Last >> |