| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Polyunsaturated fatty acid 5-lipoxygenase |
|---|
| Ligand | BDBM22323 |
|---|
| Substrate/Competitor | BDBM22319 |
|---|
| Meas. Tech. | Enzyme Inhibition Assay |
|---|
| pH | 7.4±n/a |
|---|
| Temperature | 310.15±n/a K |
|---|
| Comments | 61 +/- 1 inhibition @ 10 uM. |
|---|
| Citation |  Landwehr, J; George, S; Karg, EM; Poeckel, D; Steinhilber, D; Troschuetz, R; Werz, O Design and synthesis of novel 2-amino-5-hydroxyindole derivatives that inhibit human 5-lipoxygenase. J Med Chem49:4327-32 (2006) [PubMed] Article Landwehr, J; George, S; Karg, EM; Poeckel, D; Steinhilber, D; Troschuetz, R; Werz, O Design and synthesis of novel 2-amino-5-hydroxyindole derivatives that inhibit human 5-lipoxygenase. J Med Chem49:4327-32 (2006) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Inhibition_Run data, Solution Info, Assay Method |
|---|
| |
| Polyunsaturated fatty acid 5-lipoxygenase |
|---|
| Name: | Polyunsaturated fatty acid 5-lipoxygenase |
|---|
| Synonyms: | 5-LO | 5-Lipo-oxygenase (5-LOX) | 5-Lipoxygenase (5-LO) | 5-Lipoxygenase (LOX) | 5-Lipoygenase | 5-lipoxygenase/FLAP | ALOX5 | Arachidonate 5-lipoxygenase | LOG5 | LOX5_HUMAN |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 77972.74 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Recombinant protein was purified from E. coli lysate. After ammonium sulfate precipitation and subsequent steps, the supernatant (S100) was used for 5-LO activity assay.
|
|---|
| Residue: | 674 |
|---|
| Sequence: | MPSYTVTVATGSQWFAGTDDYIYLSLVGSAGCSEKHLLDKPFYNDFERGAVDSYDVTVDE
ELGEIQLVRIEKRKYWLNDDWYLKYITLKTPHGDYIEFPCYRWITGDVEVVLRDGRAKLA
RDDQIHILKQHRRKELETRQKQYRWMEWNPGFPLSIDAKCHKDLPRDIQFDSEKGVDFVL
NYSKAMENLFINRFMHMFQSSWNDFADFEKIFVKISNTISERVMNHWQEDLMFGYQFLNG
CNPVLIRRCTELPEKLPVTTEMVECSLERQLSLEQEVQQGNIFIVDFELLDGIDANKTDP
CTLQFLAAPICLLYKNLANKIVPIAIQLNQIPGDENPIFLPSDAKYDWLLAKIWVRSSDF
HVHQTITHLLRTHLVSEVFGIAMYRQLPAVHPIFKLLVAHVRFTIAINTKAREQLICECG
LFDKANATGGGGHVQMVQRAMKDLTYASLCFPEAIKARGMESKEDIPYYFYRDDGLLVWE
AIRTFTAEVVDIYYEGDQVVEEDPELQDFVNDVYVYGMRGRKSSGFPKSVKSREQLSEYL
TVVIFTASAQHAAVNFGQYDWCSWIPNAPPTMRAPPPTAKGVVTIEQIVDTLPDRGRSCW
HLGAVWALSQFQENELFLGMYPEEHFIEKPVKEAMARFRKNLEAIVSVIAERNKKKQLPY
YYLSPDRIPNSVAI
|
|
|
|---|
| BDBM22323 |
|---|
| BDBM22319 |
|---|
| Name | BDBM22323 |
|---|
| Synonyms: | 2-piperazinyl-5-hydroxyindole, 3i | ethyl 2-(4-benzylpiperazin-1-yl)-5-hydroxy-1H-indole-3-carboxylate |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H25N3O3 |
|---|
| Mol. Mass. | 379.4522 |
|---|
| SMILES | CCOC(=O)c1c([nH]c2ccc(O)cc12)N1CCN(Cc2ccccc2)CC1 |
|---|
| Structure | 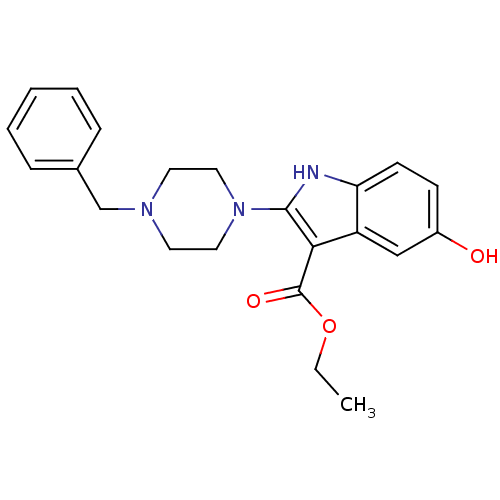
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Landwehr, J; George, S; Karg, EM; Poeckel, D; Steinhilber, D; Troschuetz, R; Werz, O Design and synthesis of novel 2-amino-5-hydroxyindole derivatives that inhibit human 5-lipoxygenase. J Med Chem49:4327-32 (2006) [PubMed] Article
Landwehr, J; George, S; Karg, EM; Poeckel, D; Steinhilber, D; Troschuetz, R; Werz, O Design and synthesis of novel 2-amino-5-hydroxyindole derivatives that inhibit human 5-lipoxygenase. J Med Chem49:4327-32 (2006) [PubMed] Article