

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM31123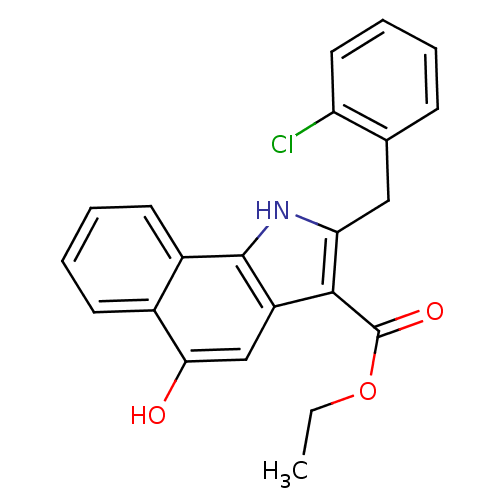 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL1beta induced human A549 cell microsomal membrane assessed as blockade of conversion of PGH2 to PGE2 | Bioorg Med Chem 17: 7924-32 (2009) Article DOI: 10.1016/j.bmc.2009.10.025 BindingDB Entry DOI: 10.7270/Q25T3KK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description Purified 5-LO was added to reaction mix containing the test compounds. For efficient inhibition of 5-LO, low hydroperoxide levels are important. Glut... | J Med Chem 49: 4327-32 (2006) Article DOI: 10.1021/jm050801i BindingDB Entry DOI: 10.7270/Q2PC30PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM31127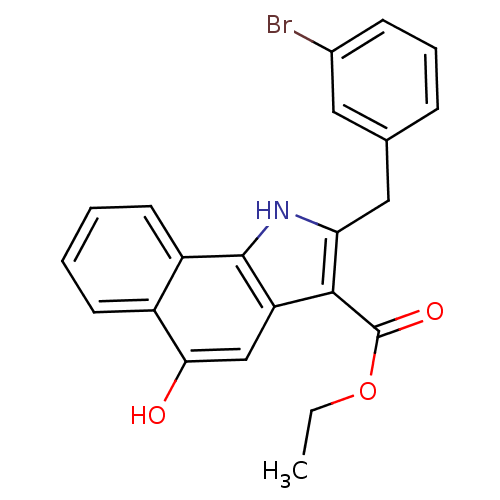 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11f) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL1beta induced human A549 cell microsomal membrane assessed as blockade of conversion of PGH2 to PGE2 | Bioorg Med Chem 17: 7924-32 (2009) Article DOI: 10.1016/j.bmc.2009.10.025 BindingDB Entry DOI: 10.7270/Q25T3KK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22332 (2-amino-5-hydroxyindole, 3n | ethyl 2-[(3-chloroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description Purified 5-LO was added to reaction mix containing the test compounds. For efficient inhibition of 5-LO, low hydroperoxide levels are important. Glut... | J Med Chem 49: 4327-32 (2006) Article DOI: 10.1021/jm050801i BindingDB Entry DOI: 10.7270/Q2PC30PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM31125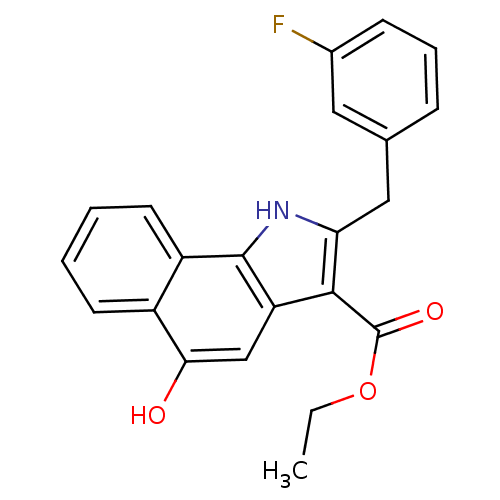 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11d) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL1beta induced human A549 cell microsomal membrane assessed as blockade of conversion of PGH2 to PGE2 | Bioorg Med Chem 17: 7924-32 (2009) Article DOI: 10.1016/j.bmc.2009.10.025 BindingDB Entry DOI: 10.7270/Q25T3KK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM31122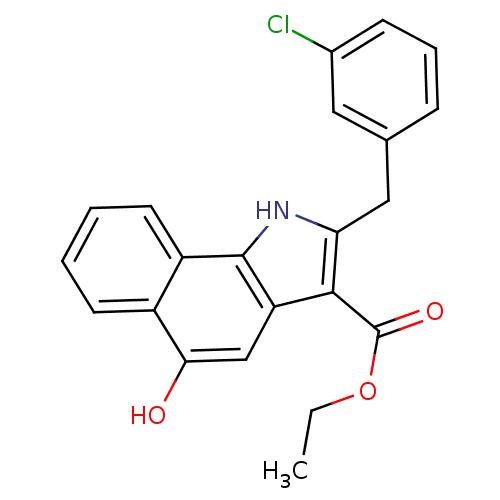 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL1beta induced human A549 cell microsomal membrane assessed as blockade of conversion of PGH2 to PGE2 | Bioorg Med Chem 17: 7924-32 (2009) Article DOI: 10.1016/j.bmc.2009.10.025 BindingDB Entry DOI: 10.7270/Q25T3KK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM31129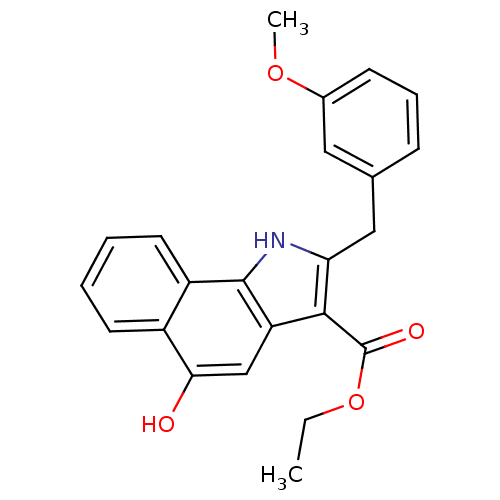 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11h) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL1beta induced human A549 cell microsomal membrane assessed as blockade of conversion of PGH2 to PGE2 | Bioorg Med Chem 17: 7924-32 (2009) Article DOI: 10.1016/j.bmc.2009.10.025 BindingDB Entry DOI: 10.7270/Q25T3KK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM31121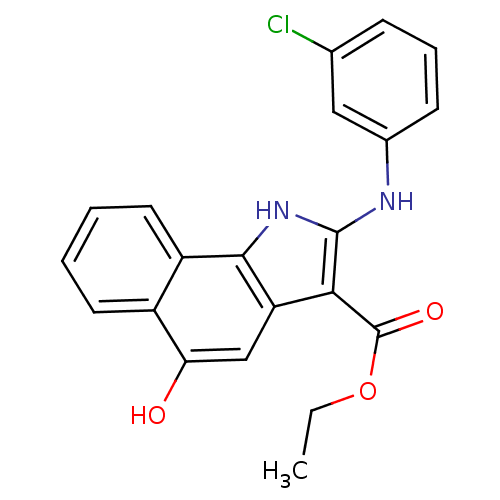 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL1beta induced human A549 cell microsomal membrane assessed as blockade of conversion of PGH2 to PGE2 | Bioorg Med Chem 17: 7924-32 (2009) Article DOI: 10.1016/j.bmc.2009.10.025 BindingDB Entry DOI: 10.7270/Q25T3KK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM31140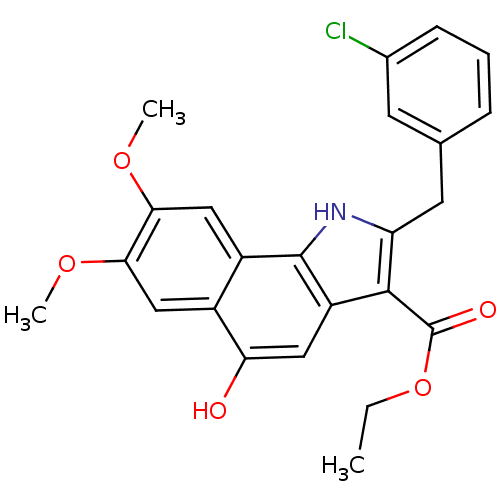 (1H-benzo[g]indole-3-carboxylate, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL1beta induced human A549 cell microsomal membrane assessed as blockade of conversion of PGH2 to PGE2 | Bioorg Med Chem 17: 7924-32 (2009) Article DOI: 10.1016/j.bmc.2009.10.025 BindingDB Entry DOI: 10.7270/Q25T3KK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL1beta induced human A549 cell microsomal membrane assessed as blockade of conversion of PGH2 to PGE2 | Bioorg Med Chem 17: 7924-32 (2009) Article DOI: 10.1016/j.bmc.2009.10.025 BindingDB Entry DOI: 10.7270/Q25T3KK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM31122 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL1beta induced human A549 cells assessed as PGE2 production preincubated for 10 mins | Bioorg Med Chem 17: 7924-32 (2009) Article DOI: 10.1016/j.bmc.2009.10.025 BindingDB Entry DOI: 10.7270/Q25T3KK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22324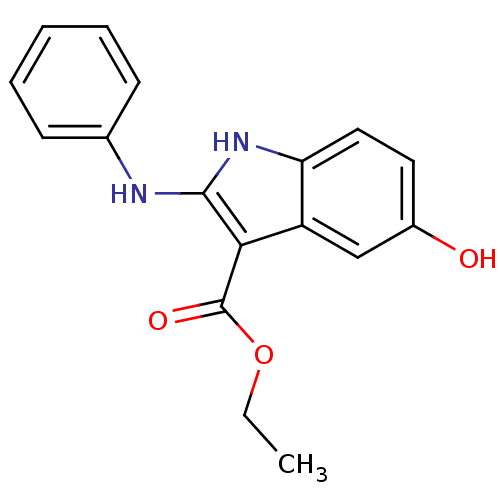 (2-amino-5-hydroxyindole, 3j | ethyl 5-hydroxy-2-(p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description Purified 5-LO was added to reaction mix containing the test compounds. For efficient inhibition of 5-LO, low hydroperoxide levels are important. Glut... | J Med Chem 49: 4327-32 (2006) Article DOI: 10.1021/jm050801i BindingDB Entry DOI: 10.7270/Q2PC30PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22322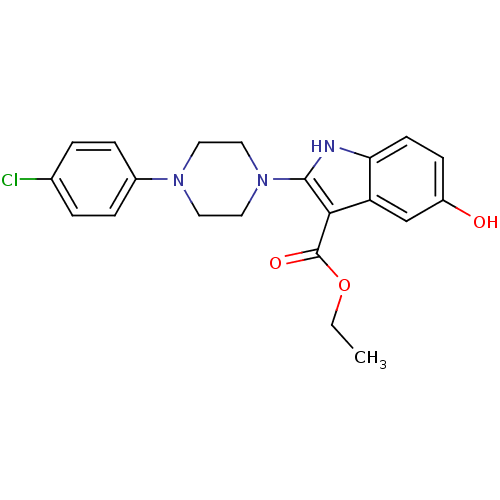 (2-piperazinyl-5-hydroxyindole, 3h | ethyl 2-[4-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description Purified 5-LO was added to reaction mix containing the test compounds. For efficient inhibition of 5-LO, low hydroperoxide levels are important. Glut... | J Med Chem 49: 4327-32 (2006) Article DOI: 10.1021/jm050801i BindingDB Entry DOI: 10.7270/Q2PC30PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50304663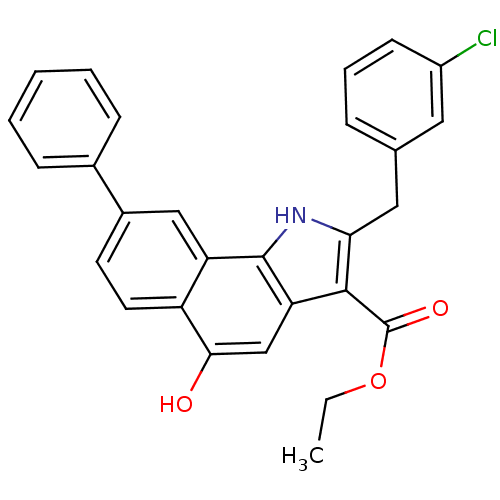 (CHEMBL595040 | ethyl 2-(3-chlorobenzyl)-5-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL1beta induced human A549 cell microsomal membrane assessed as blockade of conversion of PGH2 to PGE2 | Bioorg Med Chem 17: 7924-32 (2009) Article DOI: 10.1016/j.bmc.2009.10.025 BindingDB Entry DOI: 10.7270/Q25T3KK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22326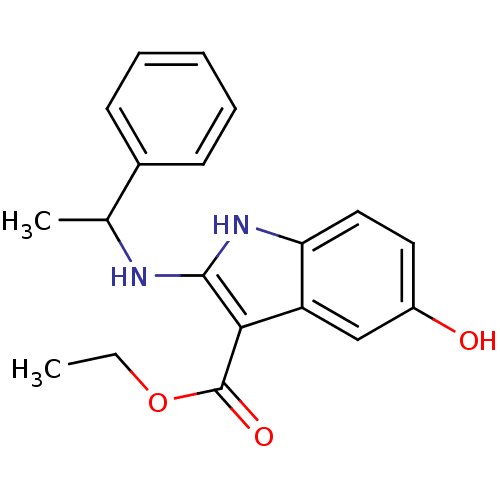 (2-amino-5-hydroxyindole, 3l | ethyl 5-hydroxy-2-[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description Purified 5-LO was added to reaction mix containing the test compounds. For efficient inhibition of 5-LO, low hydroperoxide levels are important. Glut... | J Med Chem 49: 4327-32 (2006) Article DOI: 10.1021/jm050801i BindingDB Entry DOI: 10.7270/Q2PC30PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50304664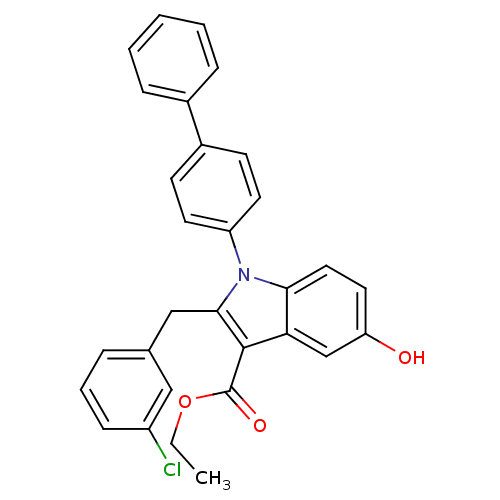 (CHEMBL605093 | ethyl 1-(biphenyl-4-yl)-2-(3-chloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL1beta induced human A549 cell microsomal membrane assessed as blockade of conversion of PGH2 to PGE2 | Bioorg Med Chem 17: 7924-32 (2009) Article DOI: 10.1016/j.bmc.2009.10.025 BindingDB Entry DOI: 10.7270/Q25T3KK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22318 (2-piperazinyl-5-hydroxyindole, 3f | ethyl 5-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description Purified 5-LO was added to reaction mix containing the test compounds. For efficient inhibition of 5-LO, low hydroperoxide levels are important. Glut... | J Med Chem 49: 4327-32 (2006) Article DOI: 10.1021/jm050801i BindingDB Entry DOI: 10.7270/Q2PC30PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM31124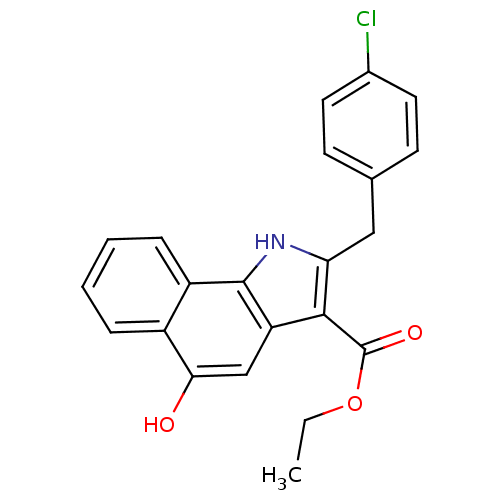 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11c) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL1beta induced human A549 cell microsomal membrane assessed as blockade of conversion of PGH2 to PGE2 | Bioorg Med Chem 17: 7924-32 (2009) Article DOI: 10.1016/j.bmc.2009.10.025 BindingDB Entry DOI: 10.7270/Q25T3KK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22325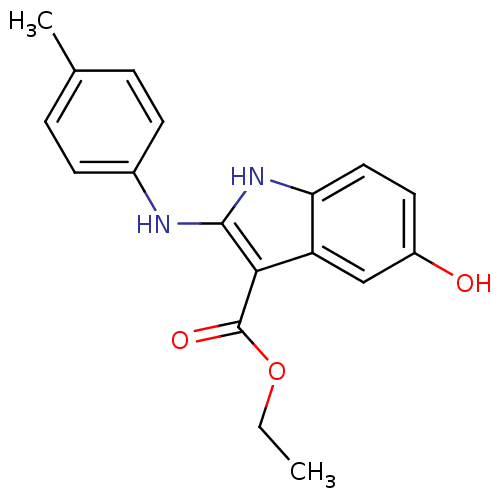 (2-amino-5-hydroxyindole, 3k | ethyl 5-hydroxy-2-[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description Purified 5-LO was added to reaction mix containing the test compounds. For efficient inhibition of 5-LO, low hydroperoxide levels are important. Glut... | J Med Chem 49: 4327-32 (2006) Article DOI: 10.1021/jm050801i BindingDB Entry DOI: 10.7270/Q2PC30PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22331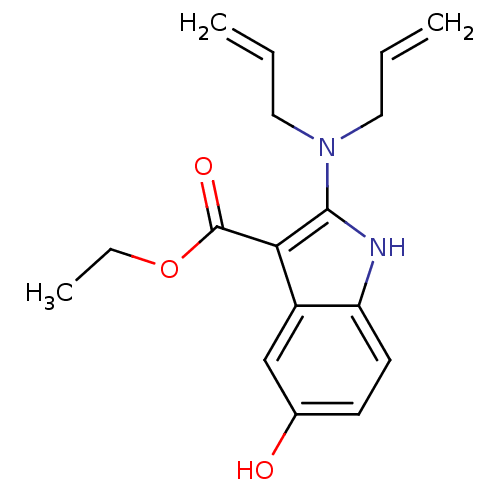 (2-amino-5-hydroxyindole, 3m | ethyl 2-[bis(prop-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description Purified 5-LO was added to reaction mix containing the test compounds. For efficient inhibition of 5-LO, low hydroperoxide levels are important. Glut... | J Med Chem 49: 4327-32 (2006) Article DOI: 10.1021/jm050801i BindingDB Entry DOI: 10.7270/Q2PC30PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM31121 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL1beta induced human A549 cells assessed as PGE2 production preincubated for 10 mins | Bioorg Med Chem 17: 7924-32 (2009) Article DOI: 10.1016/j.bmc.2009.10.025 BindingDB Entry DOI: 10.7270/Q25T3KK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM31132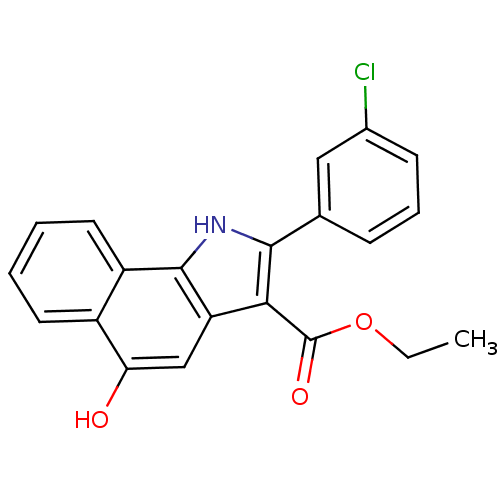 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11k) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL1beta induced human A549 cell microsomal membrane assessed as blockade of conversion of PGH2 to PGE2 | Bioorg Med Chem 17: 7924-32 (2009) Article DOI: 10.1016/j.bmc.2009.10.025 BindingDB Entry DOI: 10.7270/Q25T3KK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22329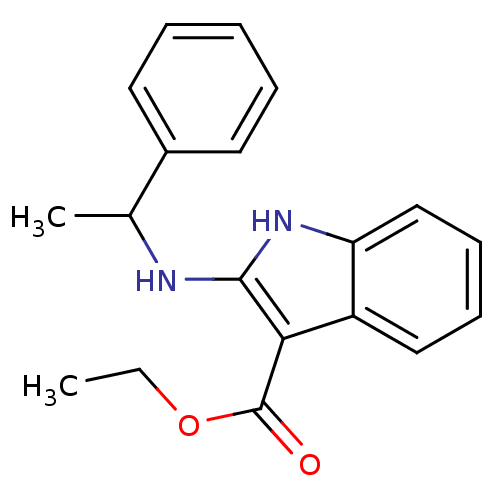 (2-aminoindole, 9b | ethyl 2-[(1-phenylethyl)amino]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description Purified 5-LO was added to reaction mix containing the test compounds. For efficient inhibition of 5-LO, low hydroperoxide levels are important. Glut... | J Med Chem 49: 4327-32 (2006) Article DOI: 10.1021/jm050801i BindingDB Entry DOI: 10.7270/Q2PC30PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22330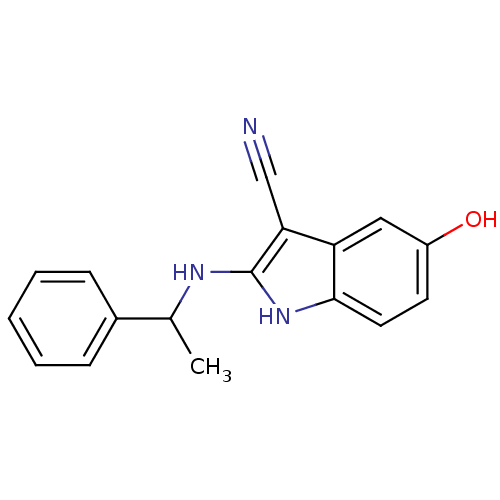 (2-amino-5-hydroxyindole, 11 | 5-hydroxy-2-[(1-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description Purified 5-LO was added to reaction mix containing the test compounds. For efficient inhibition of 5-LO, low hydroperoxide levels are important. Glut... | J Med Chem 49: 4327-32 (2006) Article DOI: 10.1021/jm050801i BindingDB Entry DOI: 10.7270/Q2PC30PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM31123 (5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL1beta induced human A549 cells assessed as PGE2 production preincubated for 10 mins | Bioorg Med Chem 17: 7924-32 (2009) Article DOI: 10.1016/j.bmc.2009.10.025 BindingDB Entry DOI: 10.7270/Q25T3KK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22320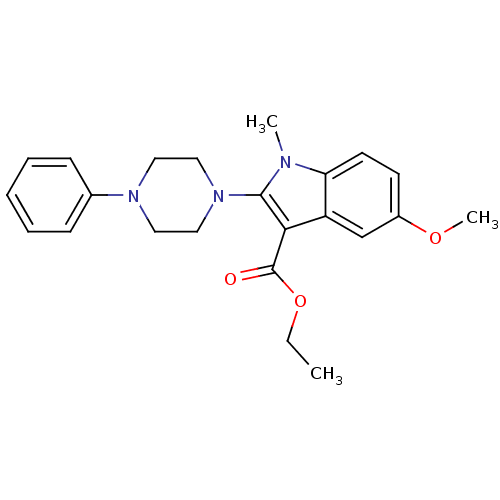 (2-piperazinyl-5-methoxyindole, 7f | ethyl 5-methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description Purified 5-LO was added to reaction mix containing the test compounds. For efficient inhibition of 5-LO, low hydroperoxide levels are important. Glut... | J Med Chem 49: 4327-32 (2006) Article DOI: 10.1021/jm050801i BindingDB Entry DOI: 10.7270/Q2PC30PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22321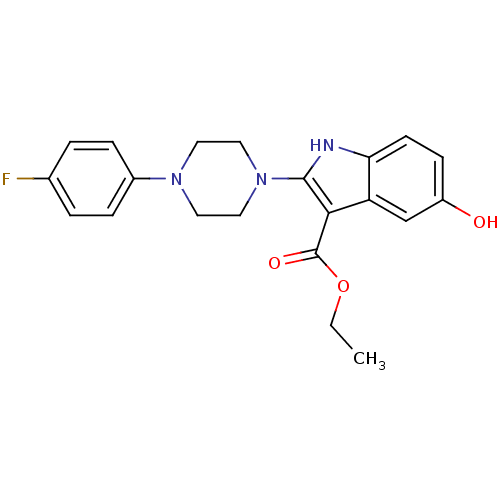 (2-piperazinyl-5-hydroxyindole, 3g | ethyl 2-[4-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description Purified 5-LO was added to reaction mix containing the test compounds. For efficient inhibition of 5-LO, low hydroperoxide levels are important. Glut... | J Med Chem 49: 4327-32 (2006) Article DOI: 10.1021/jm050801i BindingDB Entry DOI: 10.7270/Q2PC30PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22323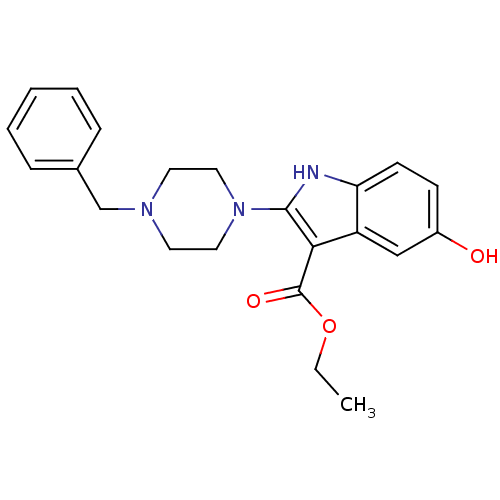 (2-piperazinyl-5-hydroxyindole, 3i | ethyl 2-(4-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description Purified 5-LO was added to reaction mix containing the test compounds. For efficient inhibition of 5-LO, low hydroperoxide levels are important. Glut... | J Med Chem 49: 4327-32 (2006) Article DOI: 10.1021/jm050801i BindingDB Entry DOI: 10.7270/Q2PC30PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22328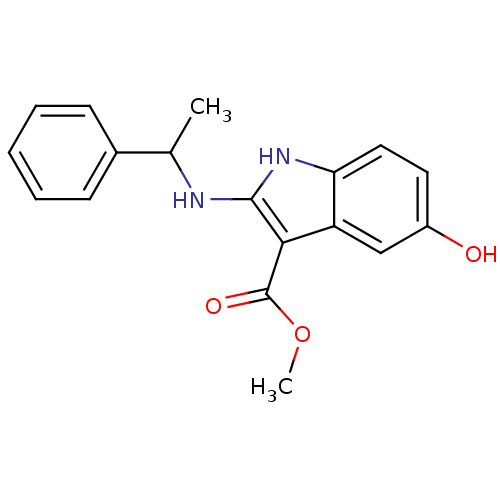 (2-amino-5-hydroxyindole, 14 | methyl 5-hydroxy-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description Purified 5-LO was added to reaction mix containing the test compounds. For efficient inhibition of 5-LO, low hydroperoxide levels are important. Glut... | J Med Chem 49: 4327-32 (2006) Article DOI: 10.1021/jm050801i BindingDB Entry DOI: 10.7270/Q2PC30PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22333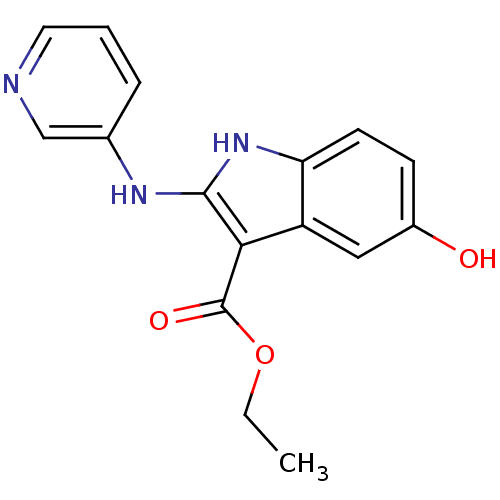 (2-amino-5-hydroxyindole, 3o | ethyl 5-hydroxy-2-(p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description Purified 5-LO was added to reaction mix containing the test compounds. For efficient inhibition of 5-LO, low hydroperoxide levels are important. Glut... | J Med Chem 49: 4327-32 (2006) Article DOI: 10.1021/jm050801i BindingDB Entry DOI: 10.7270/Q2PC30PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22327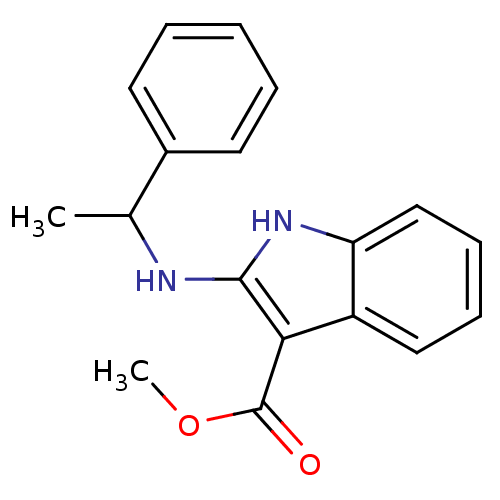 (2-aminoindole, 9a | methyl 2-[(1-phenylethyl)amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Friedrich Alexander University Erlangen | Assay Description Purified 5-LO was added to reaction mix containing the test compounds. For efficient inhibition of 5-LO, low hydroperoxide levels are important. Glut... | J Med Chem 49: 4327-32 (2006) Article DOI: 10.1021/jm050801i BindingDB Entry DOI: 10.7270/Q2PC30PQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||