| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Sucrase-isomaltase, intestinal |
|---|
| Ligand | BDBM50031483 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_88829 (CHEMBL698884) |
|---|
| IC50 | <50000±n/a nM |
|---|
| Citation |  Asano, N; Kizu, H; Oseki, K; Tomioka, E; Matsui, K; Okamoto, M; Baba, M N-alkylated nitrogen-in-the-ring sugars: conformational basis of inhibition of glycosidases and HIV-1 replication. J Med Chem38:2349-56 (1995) [PubMed] Asano, N; Kizu, H; Oseki, K; Tomioka, E; Matsui, K; Okamoto, M; Baba, M N-alkylated nitrogen-in-the-ring sugars: conformational basis of inhibition of glycosidases and HIV-1 replication. J Med Chem38:2349-56 (1995) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Sucrase-isomaltase, intestinal |
|---|
| Name: | Sucrase-isomaltase, intestinal |
|---|
| Synonyms: | SUIS_RAT | Si | Sucrase-isomaltase | alpha-Glucosidase (α-Glucosidase) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 210329.04 |
|---|
| Organism: | Rattus norvegicus (Rat) |
|---|
| Description: | P23739 |
|---|
| Residue: | 1841 |
|---|
| Sequence: | MAKKKFSALEISLIVLFIIVTAIAIALVTVLATKVPAVEEIKSPTPTSNSTPTSTPTSTS
TPTSTSTPSPGKCPPEQGEPINERINCIPEQHPTKAICEERGCCWRPWNNTVIPWCFFAD
NHGYNAESITNENAGLKATLNRIPSPTLFGEDIKSVILTTQTQTGNRFRFKITDPNNKRY
EVPHQFVKEETGIPAADTLYDVQVSENPFSIKVIRKSNNKVLCDTSVGPLLYSNQYLQIS
TRLPSEYIYGFGGHIHKRFRHDLYWKTWPIFTRDEIPGDNNHNLYGHQTFFMGIGDTSGK
SYGVFLMNSNAMEVFIQPTPIITYRVTGGILDFYIFLGDTPEQVVQQYQEVHWRPAMPAY
WNLGFQLSRWNYGSLDTVSEVVRRNREAGIPYDAQVTDIDYMEDHKEFTYDRVKFNGLPE
FAQDLHNHGKYIIILDPAISINKRANGAEYQTYVRGNEKNVWVNESDGTTPLIGEVWPGL
TVYPDFTNPQTIEWWANECNLFHQQVEYDGLWIDMNEVSSFIQGSLNLKGVLLIVLNYPP
FTPGILDKVMYSKTLCMDAVQHWGKQYDVHSLYGYSMAIATEQAVERVFPNKRSFILTRS
TFGGSGRHANHWLGDNTASWEQMEWSITGMLEFGIFGMPLVGATSCGFLADTTEELCRRW
MQLGAFYPFSRNHNAEGYMEQDPAYFGQDSSRHYLTIRYTLLPFLYTLFYRAHMFGETVA
RPFLYEFYDDTNSWIEDTQFLWGPALLITPVLRPGVENVSAYIPNATWYDYETGIKRPWR
KERINMYLPGDKIGLHLRGGYIIPTQEPDVTTTASRKNPLGLIVALDDNQAAKGELFWDD
GESKDSIEKKMYILYTFSVSNNELVLNCTHSSYAEGTSLAFKTIKVLGLREDVRSITVGE
NDQQMATHTNFTFDSANKILSITALNFNLAGSFIVRWCRTFSDNEKFTCYPDVGTATEGT
CTQRGCLWQPVSGLSNVPPYYFPPENNPYTLTSIQPLPTGITAELQLNPPNARIKLPSNP
ISTLRVGVKYHPNDMLQFKIYDAQHKRYEVPVPLNIPDTPTSSNERLYDVEIKENPFGIQ
VRRRSSGKLIWDSRLPGFGFNDQFIQISTRLPSNYLYGFGEVEHTAFKRDLNWHTWGMFT
RDQPPGYKLNSYGFHPYYMALENEGNAHGVLLLNSNGMDVTFQPTPALTYRTIGGILDFY
MFLGPTPEIATRQYHEVIGFPVMPPYWALGFQLCRYGYRNTSEIEQLYNDMVAANIPYDV
QYTDINYMERQLDFTIGERFKTLPEFVDRIRKDGMKYIVILAPAISGNETQPYPAFERGI
QKDVFVKWPNTNDICWPKVWPDLPNVTIDETITEDEAVNASRAHVAFPDFFRNSTLEWWA
REIYDFYNEKMKFDGLWIDMNEPSSFGIQMGGKVLNECRRMMTLNYPPVFSPELRVKEGE
GASISEAMCMETEHILIDGSSVLQYDVHNLYGWSQVKPTLDALQNTTGLRGIVISRSTYP
TTGRWGGHWLGDNYTTWDNLEKSLIGMLELNLFGIPYIGADICGVFHDSGYPSLYFVGIQ
VGAFYPYPRESPTINFTRSQDPVSWMKLLLQMSKKVLEIRYTLLPYFYTQMHEAHAHGGT
VIRPLMHEFFDDKETWEIYKQFLWGPAFMVTPVVEPFRTSVTGYVPKARWFDYHTGADIK
LKGILHTFSAPFDTINLHVRGGYILPCQEPARNTHLSRQNYMKLIVAADDNQMAQGTLFG
DDGESIDTYERGQYTSIQFNLNQTTLTSTVLANGYKNKQEMRLGSIHIWGKGTLRISNAN
LVYGGRKHQPPFTQEEAKETLIFDLKNMNVTLDEPIQITWS
|
|
|
|---|
| BDBM50031483 |
|---|
| n/a |
|---|
| Name | BDBM50031483 |
|---|
| Synonyms: | (2R,3R,4R,5R)-2,5-Bis-hydroxymethyl-1-methyl-pyrrolidine-3,4-diol | CHEMBL310231 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C7H15NO4 |
|---|
| Mol. Mass. | 177.1983 |
|---|
| SMILES | CN1[C@H](CO)[C@@H](O)[C@H](O)[C@H]1CO |
|---|
| Structure | 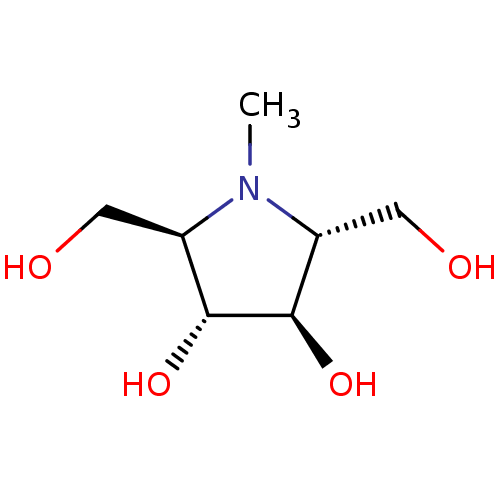
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Asano, N; Kizu, H; Oseki, K; Tomioka, E; Matsui, K; Okamoto, M; Baba, M N-alkylated nitrogen-in-the-ring sugars: conformational basis of inhibition of glycosidases and HIV-1 replication. J Med Chem38:2349-56 (1995) [PubMed]
Asano, N; Kizu, H; Oseki, K; Tomioka, E; Matsui, K; Okamoto, M; Baba, M N-alkylated nitrogen-in-the-ring sugars: conformational basis of inhibition of glycosidases and HIV-1 replication. J Med Chem38:2349-56 (1995) [PubMed]