

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-mannosidase 2 (Rattus norvegicus) | BDBM50016703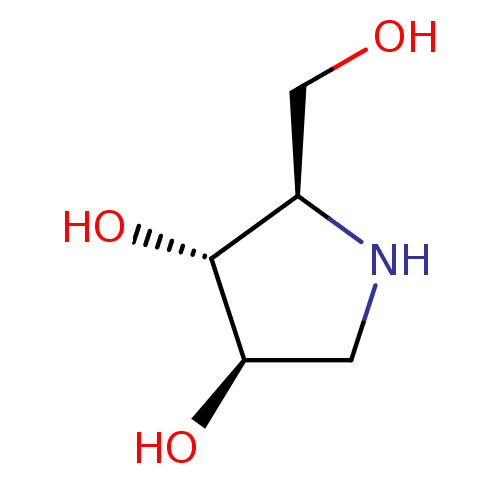 (2-Hydroxymethyl-pyrrolidine-3,4-diol | BDBM5003148...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Competitive Inhibitory activity against Golgi Alpha-mannosidase II | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Rattus norvegicus) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Competitive Inhibitory activity against Golgi Alpha-mannosidase II | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Rattus norvegicus) | BDBM18355 ((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Competitive Inhibitory activity against Golgi Alpha-mannosidase II | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Rattus norvegicus) | BDBM18353 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-methylpiperidine...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Competitive Inhibitory activity against Golgi Alpha-mannosidase II | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Rattus norvegicus) | BDBM50031480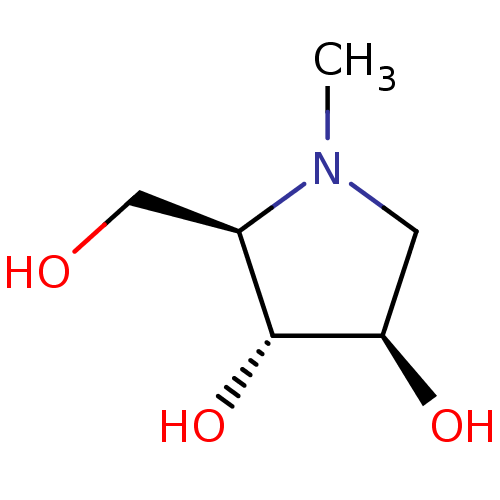 ((2R,3R,4R)-2-Hydroxymethyl-1-methyl-pyrrolidine-3,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Competitive Inhibitory activity against Golgi Alpha-mannosidase II | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Rattus norvegicus) | BDBM50031484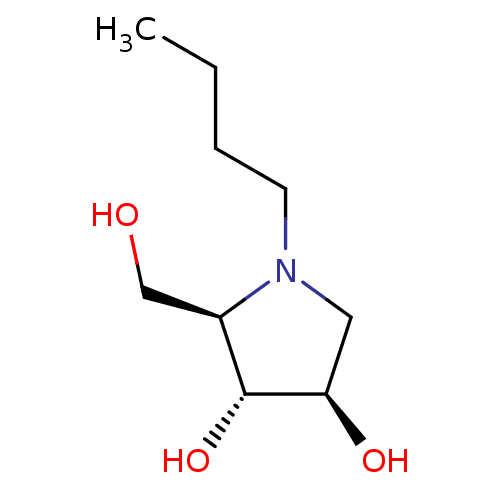 ((2R,3R,4R)-1-Butyl-2-hydroxymethyl-pyrrolidine-3,4...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Competitive Inhibitory activity against Golgi Alpha-mannosidase II | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088321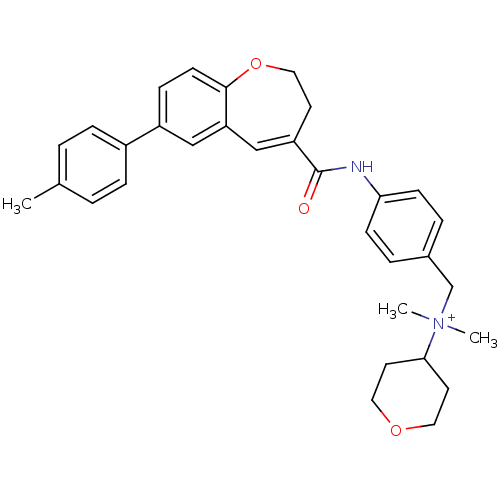 (CHEMBL292548 | Dimethyl-(tetrahydro-pyran-4-yl)-{4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088302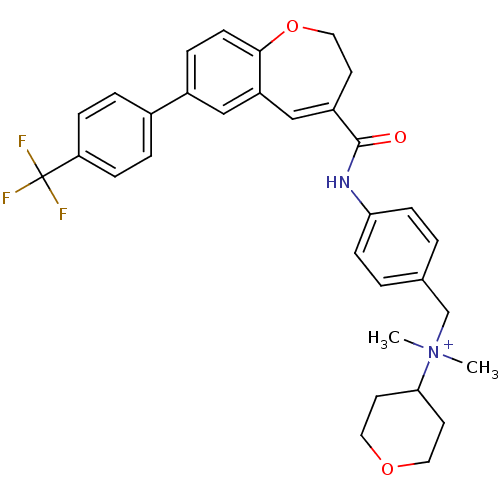 (CHEMBL56565 | Dimethyl-(tetrahydro-pyran-4-yl)-(4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088322 ((4-{[7-(4-Ethoxy-phenyl)-2,3-dihydro-benzo[b]oxepi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088319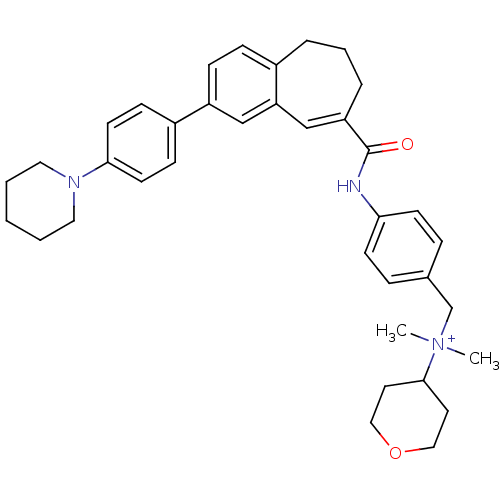 (CHEMBL62339 | Dimethyl-(4-{[3-(4-piperidin-1-yl-ph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064157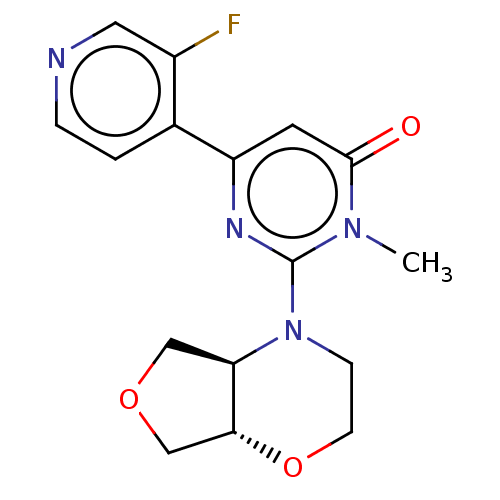 (CHEMBL3401132) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088311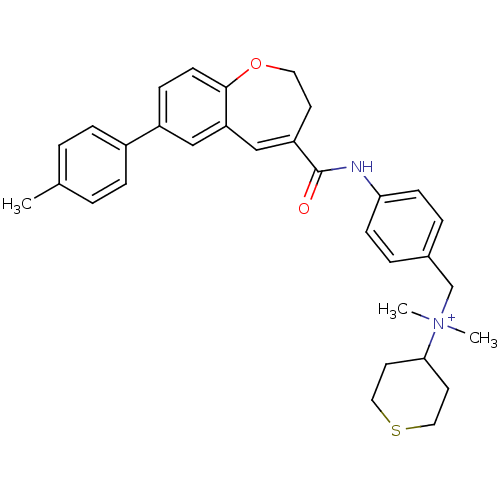 (CHEMBL61208 | Dimethyl-(tetrahydro-thiopyran-4-yl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088306 ((1-Ethyl-propyl)-dimethyl-{4-[(7-p-tolyl-2,3-dihyd...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064156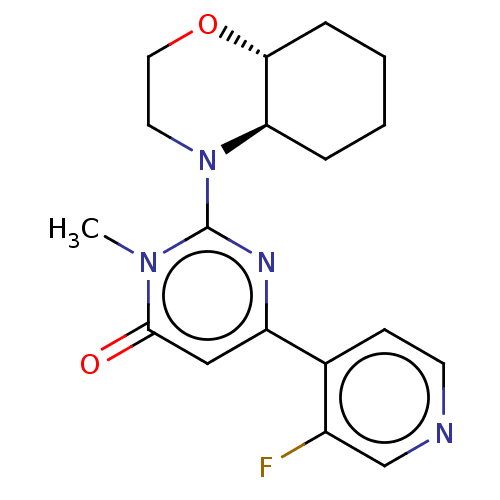 (CHEMBL3401131) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088312 (CHEMBL62180 | Dimethyl-(4-{[3-(4-pyrrolidin-1-yl-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064154 (CHEMBL3401129) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088325 (CHEMBL62598 | Dimethyl-(4-oxo-cyclohexyl)-{4-[(7-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064152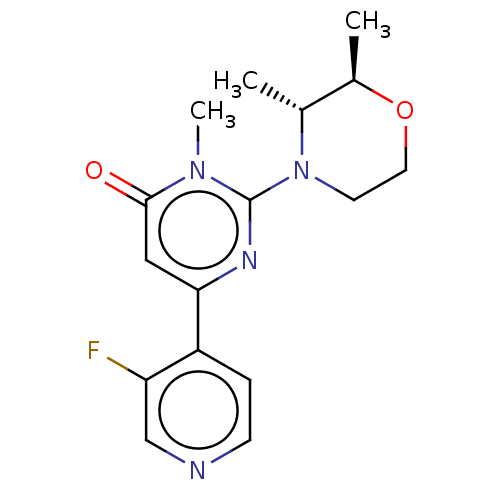 (CHEMBL3401127) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088315 ((3-Hydroxy-propyl)-dimethyl-{4-[(7-p-tolyl-2,3-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064143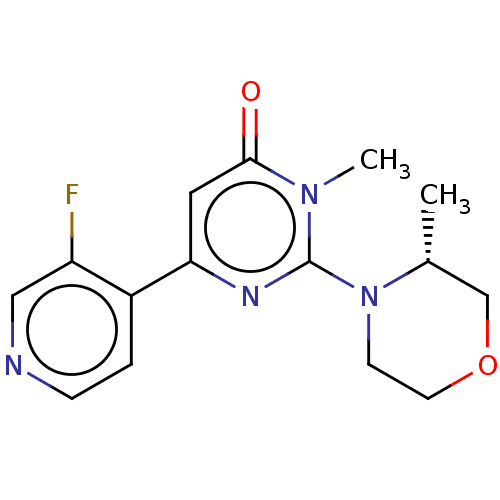 (CHEMBL3401118) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064153 (CHEMBL3401128) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50443871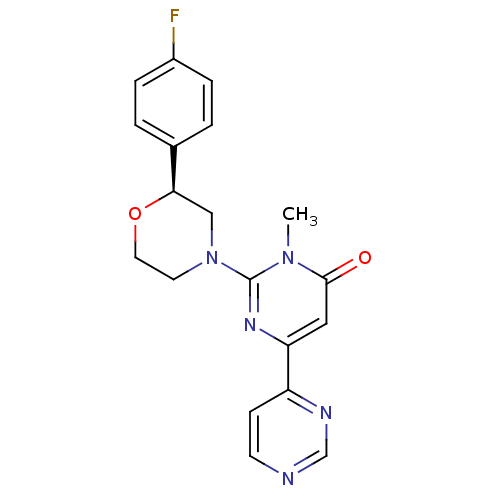 (CHEMBL3091536) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064142 (CHEMBL3401117) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064141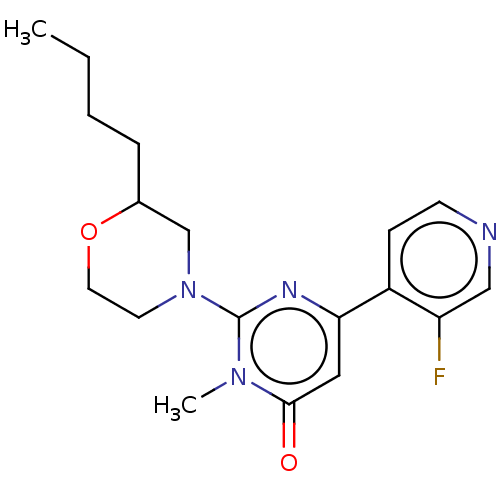 (CHEMBL3401116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064145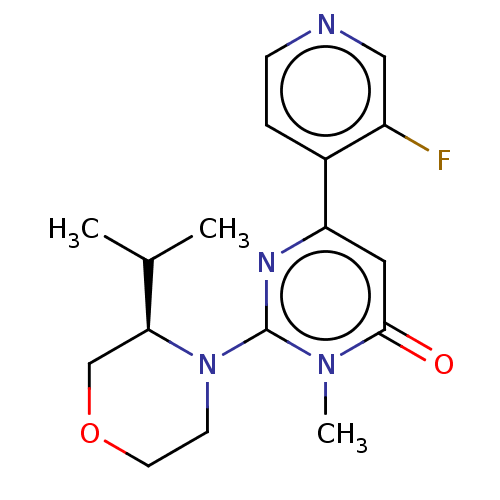 (CHEMBL3401120) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM50334268 (CHEMBL1642655 | CHEMBL2205637 | N-(6-(1H-imidazol-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of ASK1 assessed as phosphorylated fluorescent peptide by mobility shift assay | Bioorg Med Chem 19: 486-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.004 BindingDB Entry DOI: 10.7270/Q2JH3MGM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM50334269 (4-tert-butyl-N-(imidazo[1,2-a]quinolin-2-yl)benzam...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of ASK1 assessed as phosphorylated fluorescent peptide by mobility shift assay | Bioorg Med Chem 19: 486-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.004 BindingDB Entry DOI: 10.7270/Q2JH3MGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064150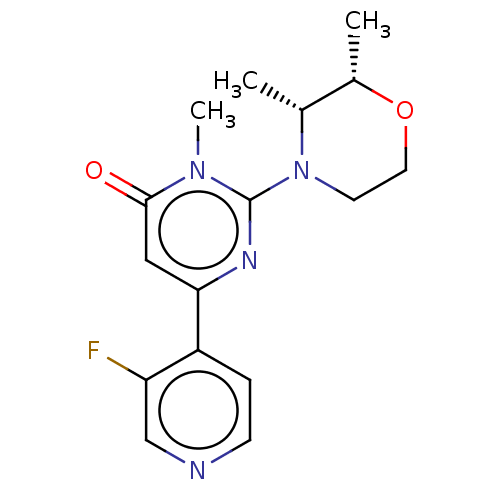 (CHEMBL3401125) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064150 (CHEMBL3401125) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064140 (CHEMBL3401115) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064144 (CHEMBL3401119) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064151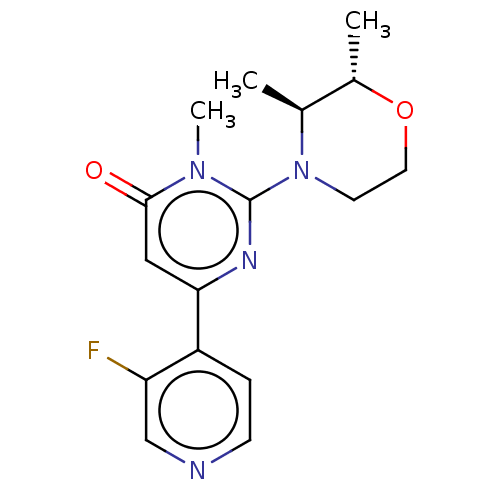 (CHEMBL3401126) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM50334270 (2-methyl-2-(4-(6-(thiophen-3-yl)imidazo[1,2-a]pyri...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of ASK1 assessed as phosphorylated fluorescent peptide by mobility shift assay | Bioorg Med Chem 19: 486-9 (2011) Article DOI: 10.1016/j.bmc.2010.11.004 BindingDB Entry DOI: 10.7270/Q2JH3MGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064146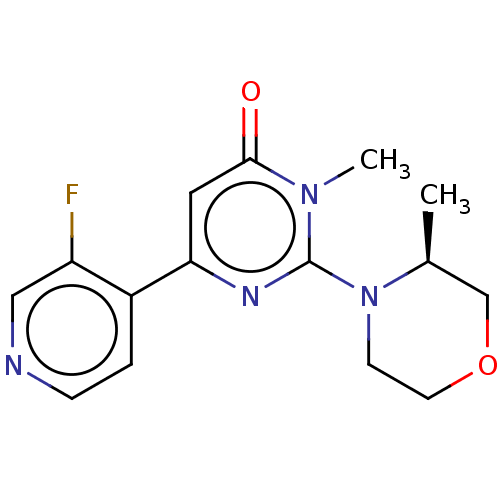 (CHEMBL3401121) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088320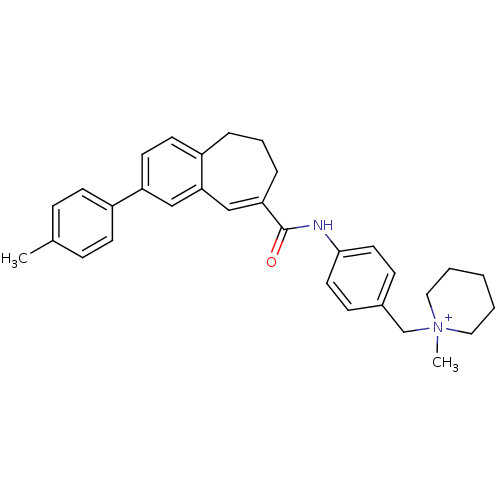 (1-Methyl-1-{4-[(3-p-tolyl-8,9-dihydro-7H-benzocycl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect of the compound on the binding of [125I]- MCP-1 to C-C chemokine receptor type 2-expressing CHO cells | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064147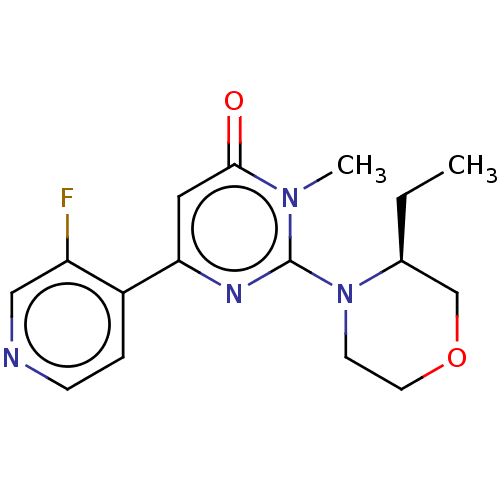 (CHEMBL3401122) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064155 (CHEMBL3401130) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM18353 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-methylpiperidine...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of Sucrase in rat intestinal brush border membranes by D-glucose oxidase-peroxidase method | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM18353 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-methylpiperidine...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of Sucrase in rat intestinal brush border membranes by D-glucose oxidase-peroxidase method | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064149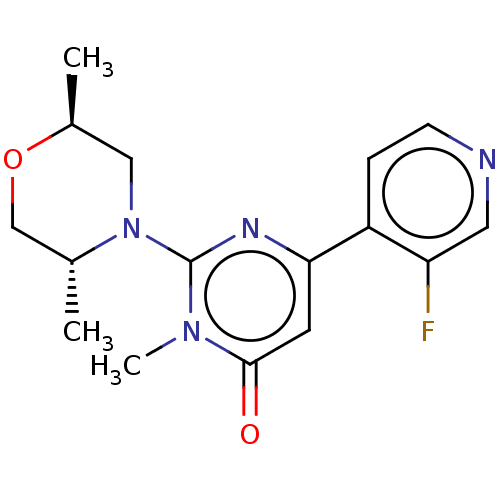 (CHEMBL3401124) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088309 (1-Methyl-1-{4-[(7-p-tolyl-2,3-dihydro-benzo[b]oxep...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES | J Med Chem 43: 2049-63 (2000) BindingDB Entry DOI: 10.7270/Q26D5S75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50344203 (5-Amino-3-[[3-(3-aminophenyl)-5-methyl-1H-pyrrol-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of ALK using FL-Peptide 13, 5-FAM-KKSRGDYMTMQIG-CONH2 substrate after 60 mins by mobility shift assay | Bioorg Med Chem 19: 3086-95 (2011) Article DOI: 10.1016/j.bmc.2011.04.008 BindingDB Entry DOI: 10.7270/Q2JQ11B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50344205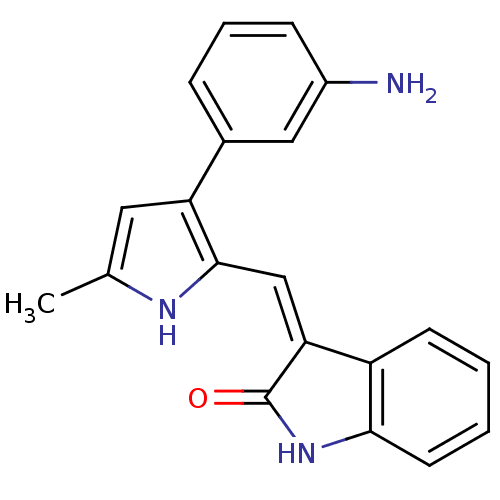 (3-[[3-(3-Aminophenyl)-5-methyl-1H-pyrrol-2-yl]meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of ALK using FL-Peptide 13, 5-FAM-KKSRGDYMTMQIG-CONH2 substrate after 60 mins by mobility shift assay | Bioorg Med Chem 19: 3086-95 (2011) Article DOI: 10.1016/j.bmc.2011.04.008 BindingDB Entry DOI: 10.7270/Q2JQ11B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064139 (CHEMBL3401114) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50344202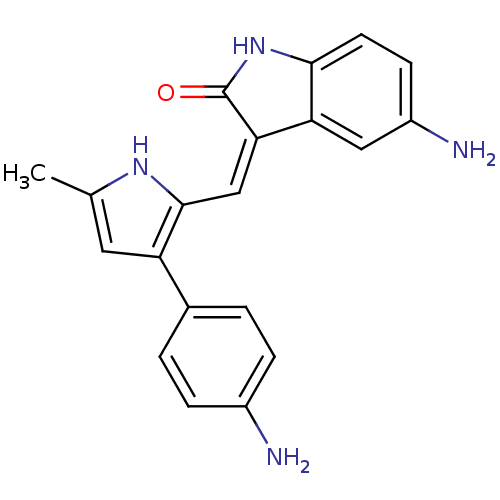 (5-Amino-3-[[3-(4-aminophenyl)-5-methyl-1H-pyrrol-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaDesign, Inc. Curated by ChEMBL | Assay Description Inhibition of ALK using FL-Peptide 13, 5-FAM-KKSRGDYMTMQIG-CONH2 substrate after 60 mins by mobility shift assay | Bioorg Med Chem 19: 3086-95 (2011) Article DOI: 10.1016/j.bmc.2011.04.008 BindingDB Entry DOI: 10.7270/Q2JQ11B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 244 total ) | Next | Last >> |