| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acyl-CoA:cholesterol acyltransferase |
|---|
| Ligand | BDBM50047546 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_28332 |
|---|
| IC50 | 48±n/a nM |
|---|
| Citation |  Kimura, T; Watanabe, N; Matsui, M; Hayashi, K; Tanaka, H; Ohtsuka, I; Saeki, T; Kogushi, M; Kabayashi, H; Akasaka, K Structure-activity relationship of a series of phenylureas linked to 4-phenylimidazole. Novel potent inhibitors of acyl-CoA:cholesterol O-acyltransferase with antiatherosclerotic activity. 2. J Med Chem36:1641-53 (1993) [PubMed] Kimura, T; Watanabe, N; Matsui, M; Hayashi, K; Tanaka, H; Ohtsuka, I; Saeki, T; Kogushi, M; Kabayashi, H; Akasaka, K Structure-activity relationship of a series of phenylureas linked to 4-phenylimidazole. Novel potent inhibitors of acyl-CoA:cholesterol O-acyltransferase with antiatherosclerotic activity. 2. J Med Chem36:1641-53 (1993) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acyl-CoA:cholesterol acyltransferase |
|---|
| Name: | Acyl-CoA:cholesterol acyltransferase |
|---|
| Synonyms: | ACAT |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 35405.31 |
|---|
| Organism: | Oryctolagus cuniculus |
|---|
| Description: | n/a |
|---|
| Residue: | 305 |
|---|
| Sequence: | PLFLKEVGSHFDDFVTNLIEKSASLDNGGCALTTFSILKEMKNNHRAKDLRAPPEQGKIF
VARRSLLDELFEVDHIRTIYHMFIALLILFILSTLVVDYIDEGRLVLEFNLLSYAFGKLP
TVVWTWWTMFLSTLSIPYFLFQHWANGYSKSSHPLMYSLFHGLLFMVFQLGILGFGPTYI
VLAYTLPPASRFIVILEQIRLIMKAHSFVRENVPRVLNSAKEKSSTVPIPTVNQYLYFLF
APTLIYRDSYPRTPTVRWGYVAMQFAQVFGCLFYVYYIFERLCAPLFRNIKQEPFSARVL
VLCIF
|
|
|
|---|
| BDBM50047546 |
|---|
| n/a |
|---|
| Name | BDBM50047546 |
|---|
| Synonyms: | 1-{2-[3-(5-Chloro-4-phenyl-imidazol-1-yl)-propoxy]-6-methyl-phenyl}-3-(2,2-dimethyl-propyl)-urea | CHEMBL44848 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H31ClN4O2 |
|---|
| Mol. Mass. | 454.992 |
|---|
| SMILES | Cc1cccc(OCCCn2cnc(c2Cl)-c2ccccc2)c1NC(=O)NCC(C)(C)C |
|---|
| Structure | 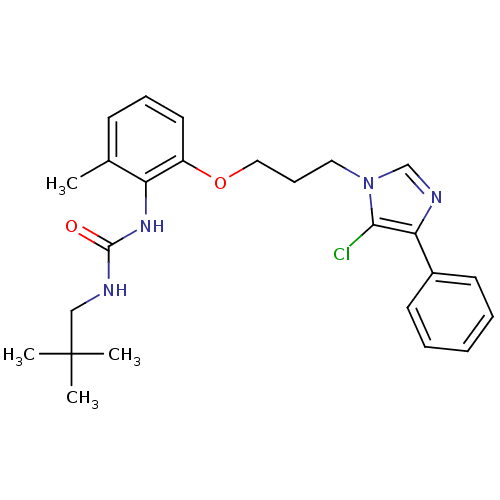
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kimura, T; Watanabe, N; Matsui, M; Hayashi, K; Tanaka, H; Ohtsuka, I; Saeki, T; Kogushi, M; Kabayashi, H; Akasaka, K Structure-activity relationship of a series of phenylureas linked to 4-phenylimidazole. Novel potent inhibitors of acyl-CoA:cholesterol O-acyltransferase with antiatherosclerotic activity. 2. J Med Chem36:1641-53 (1993) [PubMed]
Kimura, T; Watanabe, N; Matsui, M; Hayashi, K; Tanaka, H; Ohtsuka, I; Saeki, T; Kogushi, M; Kabayashi, H; Akasaka, K Structure-activity relationship of a series of phenylureas linked to 4-phenylimidazole. Novel potent inhibitors of acyl-CoA:cholesterol O-acyltransferase with antiatherosclerotic activity. 2. J Med Chem36:1641-53 (1993) [PubMed]