| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Chymotrypsin-C |
|---|
| Ligand | BDBM50090238 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_197815 |
|---|
| Ki | 12200±n/a nM |
|---|
| Citation |  Hayler, J; Kane, PD; LeGrand, D; Lugrin, F; Menear, K; Price, R; Allen, M; Cockcroft, X; Ambler, J; Butler, K; Dunnet, K; Mitchelson, A; Talbot, M; Tweed, M; Wills, N The design and synthesis of thrombin inhibitors: the introduction of in vivo efficacy and oral bioavailability into benzthiazolylalanine inhibitors. Bioorg Med Chem Lett10:1567-70 (2000) [PubMed] Hayler, J; Kane, PD; LeGrand, D; Lugrin, F; Menear, K; Price, R; Allen, M; Cockcroft, X; Ambler, J; Butler, K; Dunnet, K; Mitchelson, A; Talbot, M; Tweed, M; Wills, N The design and synthesis of thrombin inhibitors: the introduction of in vivo efficacy and oral bioavailability into benzthiazolylalanine inhibitors. Bioorg Med Chem Lett10:1567-70 (2000) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Chymotrypsin-C |
|---|
| Name: | Chymotrypsin-C |
|---|
| Synonyms: | CLCR | CTRC | CTRC_HUMAN | Caldecrin | Chymotrypsin | Chymotrypsin C | Chymotrypsin-C |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 29487.98 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q99895 |
|---|
| Residue: | 268 |
|---|
| Sequence: | MLGITVLAALLACASSCGVPSFPPNLSARVVGGEDARPHSWPWQISLQYLKNDTWRHTCG
GTLIASNFVLTAAHCISNTRTYRVAVGKNNLEVEDEEGSLFVGVDTIHVHKRWNALLLRN
DIALIKLAEHVELSDTIQVACLPEKDSLLPKDYPCYVTGWGRLWTNGPIADKLQQGLQPV
VDHATCSRIDWWGFRVKKTMVCAGGDGVISACNGDSGGPLNCQLENGSWEVFGIVSFGSR
RGCNTRKKPVVYTRVSAYIDWINEKMQL
|
|
|
|---|
| BDBM50090238 |
|---|
| n/a |
|---|
| Name | BDBM50090238 |
|---|
| Synonyms: | 3,3-Dimethyl-6-{3-[4-(2-morpholin-4-yl-2-oxo-ethyl)-piperazin-1-yl]-3-oxo-propyl}-1,2,3,4-tetrahydro-quinoline-8-sulfonic acid {(S)-1-benzothiazol-2-ylmethyl-2-[4-(2-fluoro-ethyl)-piperidin-1-yl]-2-oxo-ethyl}-amide | CHEMBL263806 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C41H56FN7O6S2 |
|---|
| Mol. Mass. | 826.055 |
|---|
| SMILES | CC1(C)CNc2c(C1)cc(CCC(=O)N1CCN(CC(=O)N3CCOCC3)CC1)cc2S(=O)(=O)N[C@@H](Cc1nc2ccccc2s1)C(=O)N1CCC(CCF)CC1 |
|---|
| Structure | 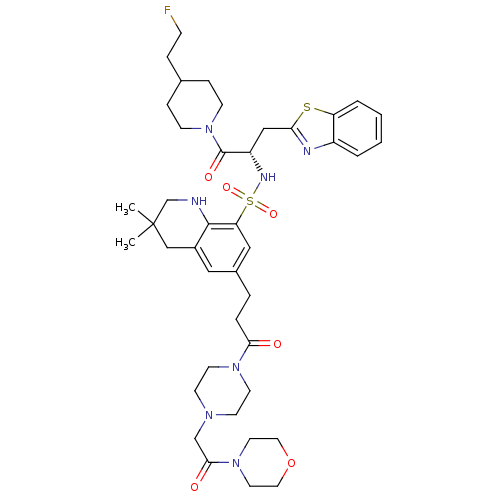
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Hayler, J; Kane, PD; LeGrand, D; Lugrin, F; Menear, K; Price, R; Allen, M; Cockcroft, X; Ambler, J; Butler, K; Dunnet, K; Mitchelson, A; Talbot, M; Tweed, M; Wills, N The design and synthesis of thrombin inhibitors: the introduction of in vivo efficacy and oral bioavailability into benzthiazolylalanine inhibitors. Bioorg Med Chem Lett10:1567-70 (2000) [PubMed]
Hayler, J; Kane, PD; LeGrand, D; Lugrin, F; Menear, K; Price, R; Allen, M; Cockcroft, X; Ambler, J; Butler, K; Dunnet, K; Mitchelson, A; Talbot, M; Tweed, M; Wills, N The design and synthesis of thrombin inhibitors: the introduction of in vivo efficacy and oral bioavailability into benzthiazolylalanine inhibitors. Bioorg Med Chem Lett10:1567-70 (2000) [PubMed]