

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50090249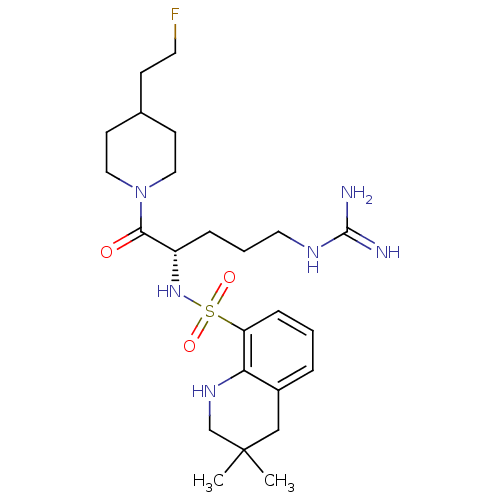 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090249 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090222 (CHEMBL39086 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50077058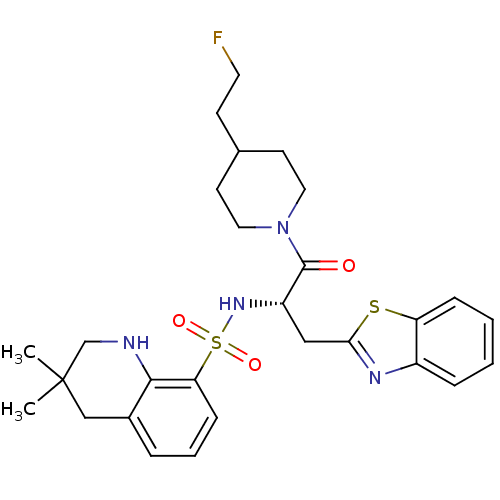 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090244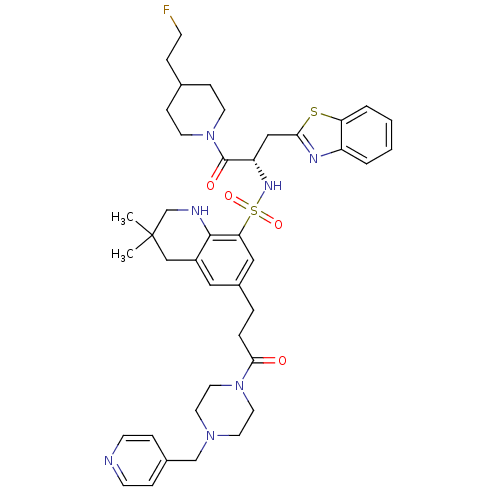 (3,3-Dimethyl-6-[3-oxo-3-(4-pyridin-4-ylmethyl-pipe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090222 (CHEMBL39086 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090250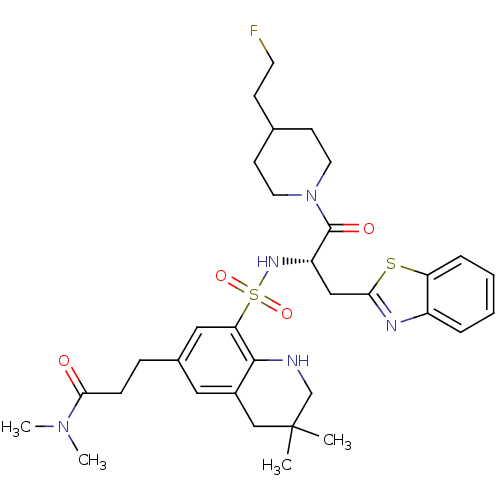 (3-(8-{(S)-1-Benzothiazol-2-ylmethyl-2-[4-(2-fluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090247 (6-[3-(4-Acetyl-piperazin-1-yl)-3-oxo-propyl]-3,3-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090243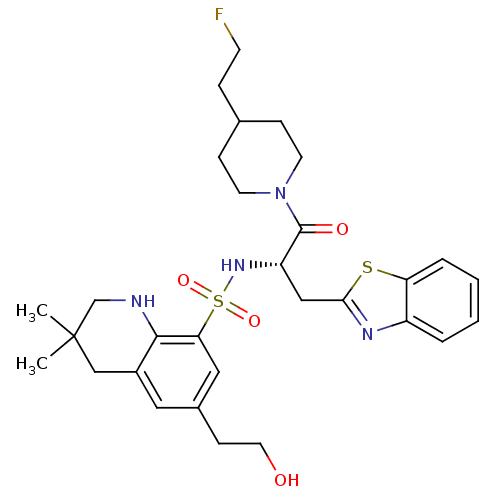 (6-(2-Hydroxy-ethyl)-3,3-dimethyl-1,2,3,4-tetrahydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090246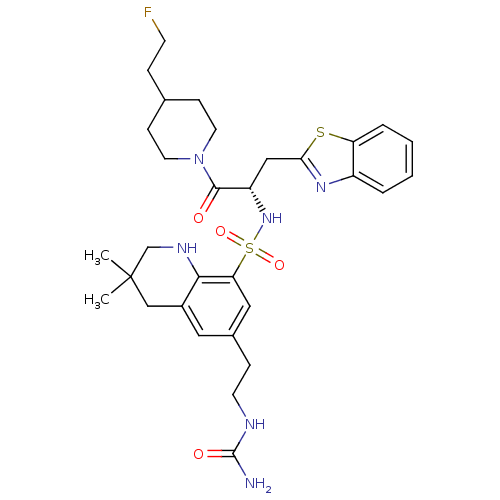 (3,3-Dimethyl-6-(2-ureido-ethyl)-1,2,3,4-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090238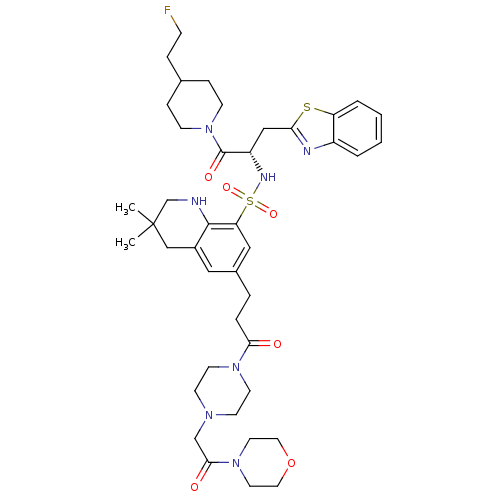 (3,3-Dimethyl-6-{3-[4-(2-morpholin-4-yl-2-oxo-ethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090245 (6-Hydroxymethyl-3,3-dimethyl-1,2,3,4-tetrahydro-qu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090248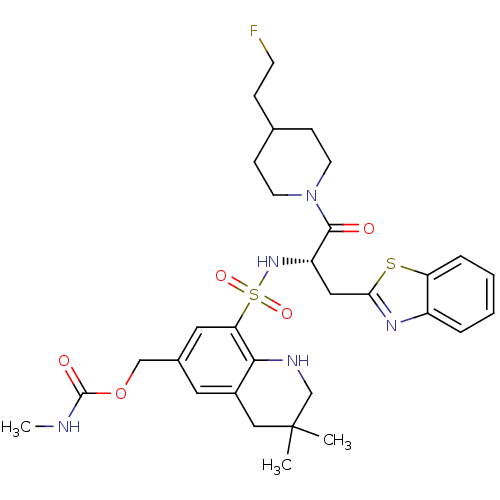 (CHEMBL43397 | Methyl-carbamic acid 8-{(S)-1-benzot...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090241 (3-(8-{(S)-1-Benzothiazol-2-ylmethyl-2-[4-(2-fluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090242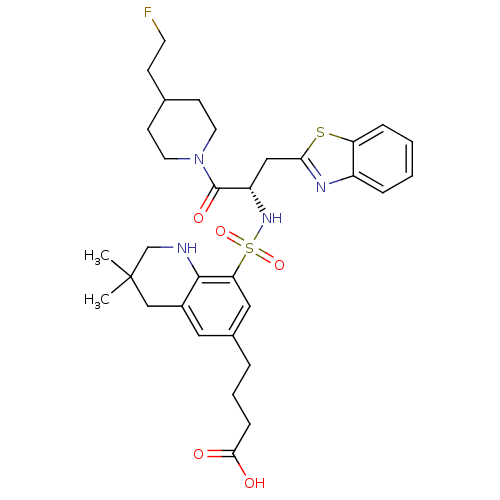 (4-(8-{(S)-1-Benzothiazol-2-ylmethyl-2-[4-(2-fluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090226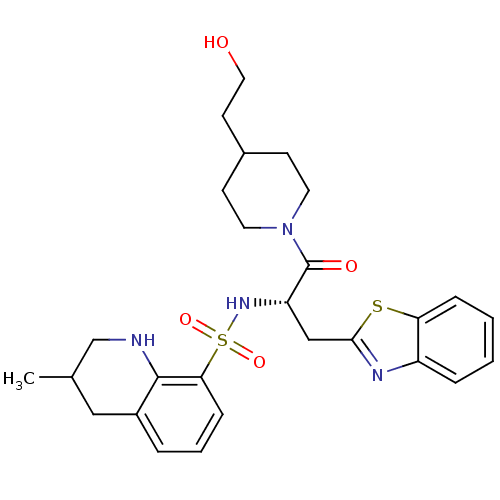 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090226 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090253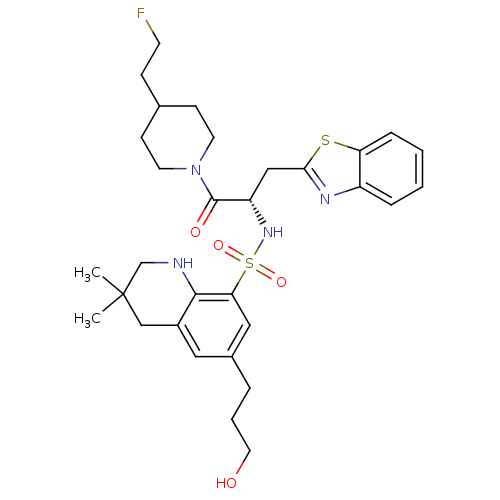 (6-(3-Hydroxy-propyl)-3,3-dimethyl-1,2,3,4-tetrahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090251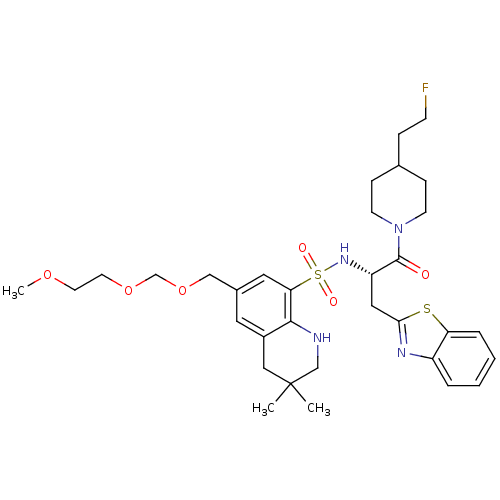 (6-(2-Methoxy-ethoxymethoxymethyl)-3,3-dimethyl-1,2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50366643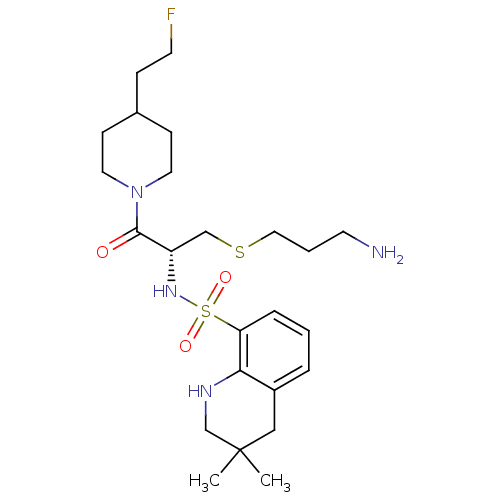 (CHEMBL1907778) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090231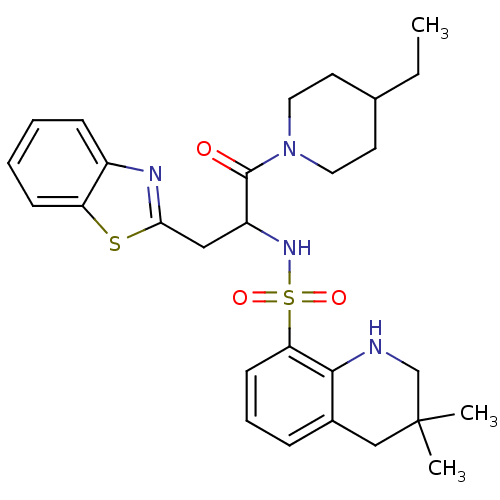 (CHEMBL39375 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50366642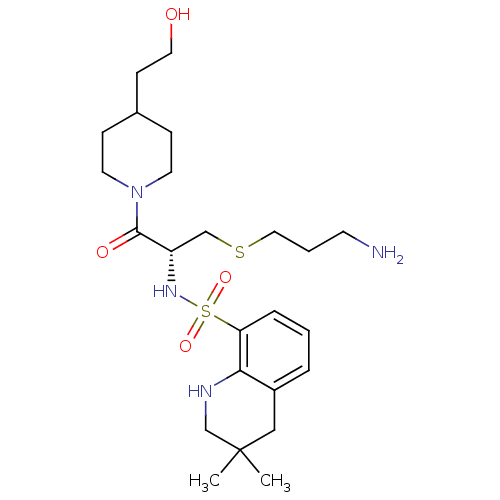 (CHEMBL1907779) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090240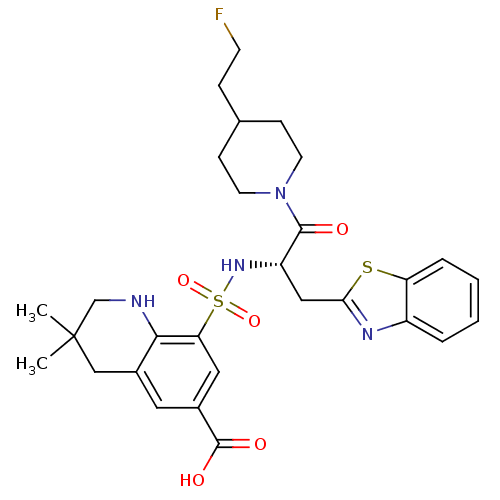 (8-{(S)-1-Benzothiazol-2-ylmethyl-2-[4-(2-fluoro-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 359 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090236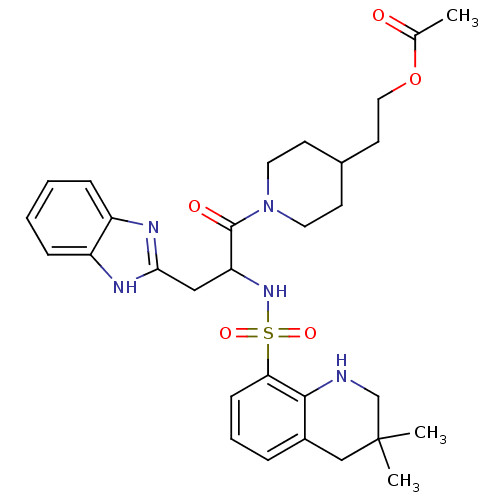 (CHEMBL418248 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 452 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090239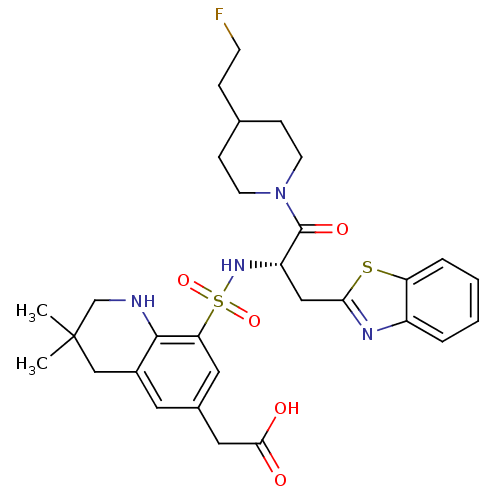 ((8-{(S)-1-Benzothiazol-2-ylmethyl-2-[4-(2-fluoro-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090252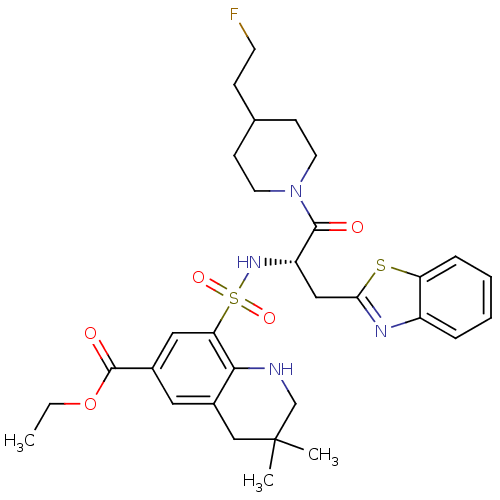 (8-{(S)-1-Benzothiazol-2-ylmethyl-2-[4-(2-fluoro-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 723 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090235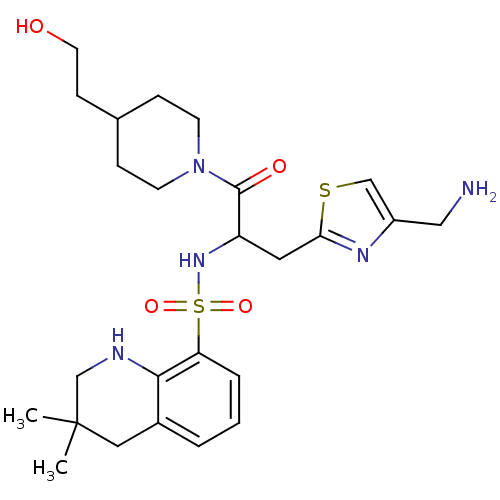 (CHEMBL38907 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090237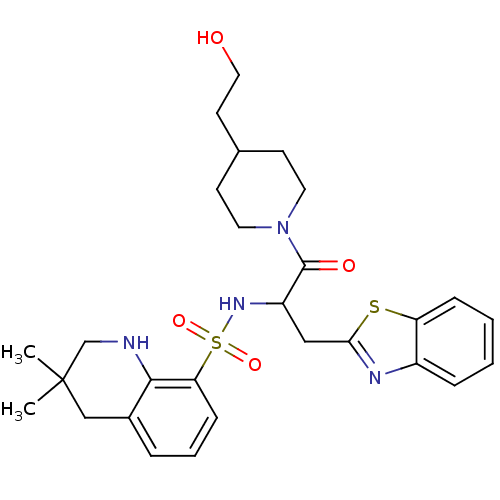 (CHEMBL43074 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090220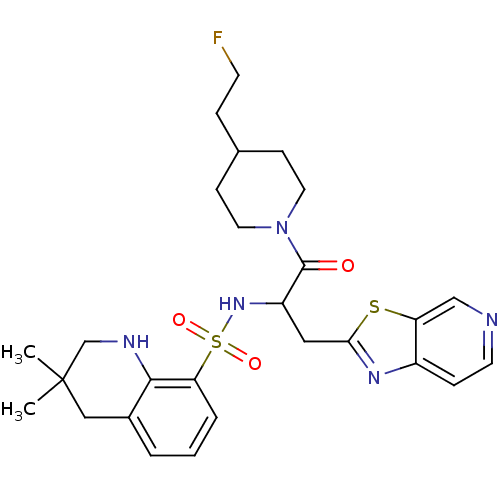 (CHEMBL289623 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50366643 (CHEMBL1907778) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Trypsin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090234 (CHEMBL40904 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50366642 (CHEMBL1907779) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Trypsin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090230 (CHEMBL38617 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090224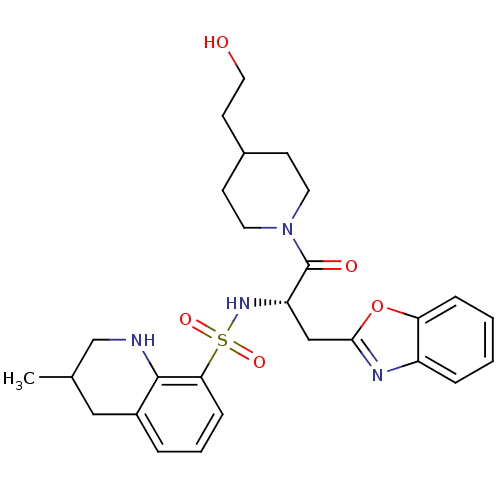 (CHEMBL289493 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090227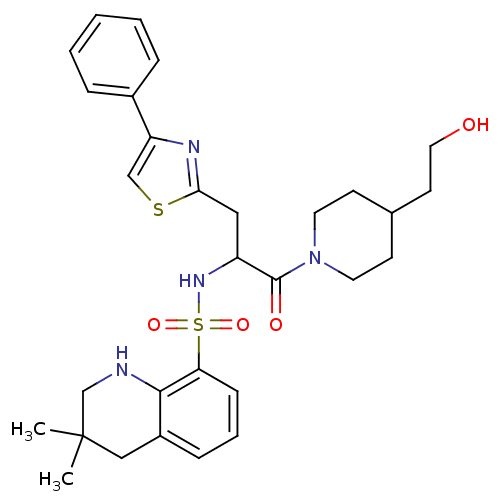 (CHEMBL288938 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50366643 (CHEMBL1907778) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against plasmin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090228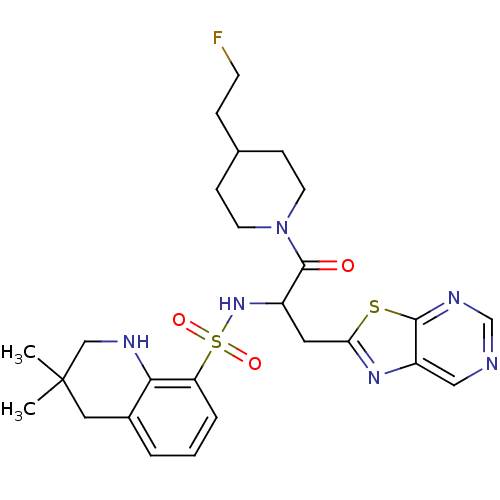 (CHEMBL289628 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50090231 (CHEMBL39375 | MD805 Analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Serine protease chymotrypsin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50366642 (CHEMBL1907779) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against plasmin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090225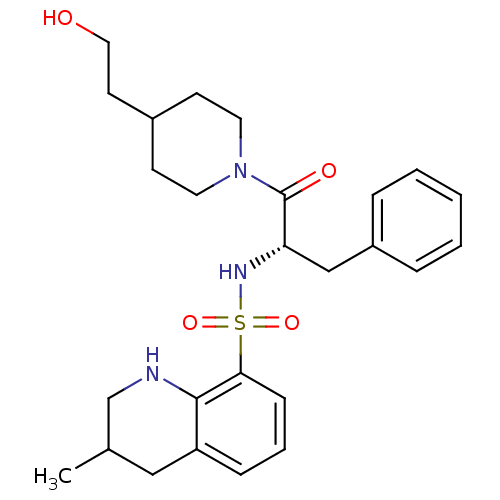 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50090226 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Serine protease chymotrypsin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50090222 (CHEMBL39086 | MD805 Analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Serine protease chymotrypsin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50366643 (CHEMBL1907778) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Serine protease chymotrypsin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50090238 (3,3-Dimethyl-6-{3-[4-(2-morpholin-4-yl-2-oxo-ethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against Serine protease chymotrypsin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50366644 (CHEMBL1907777) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50090226 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Coagulation factor X | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM50090226 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against kallikrein | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090223 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090232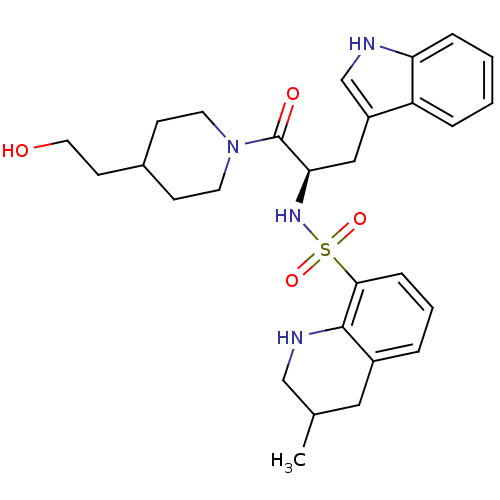 (CHEMBL40319 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50366642 (CHEMBL1907779) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Coagulation factor X | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 63 total ) | Next | Last >> |