

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM50156495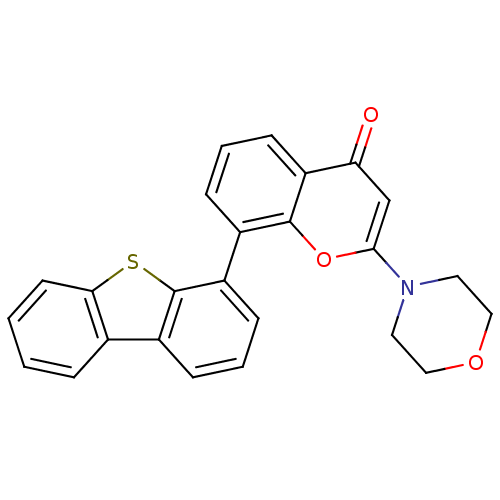 (8-(dibenzo[b,d]thiophen-4-yl)-2-morpholino-4H-chro...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Natural Sciences--Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against DNA-dependent protein kinase receptor | J Med Chem 48: 7829-46 (2005) Article DOI: 10.1021/jm050444b BindingDB Entry DOI: 10.7270/Q2Z31Z60 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090249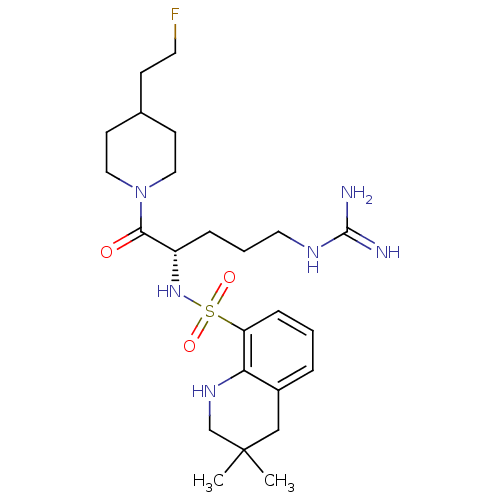 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090249 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50077057 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of human thrombin (in vitro) | Bioorg Med Chem Lett 9: 1317-22 (1999) BindingDB Entry DOI: 10.7270/Q2ZW1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50077058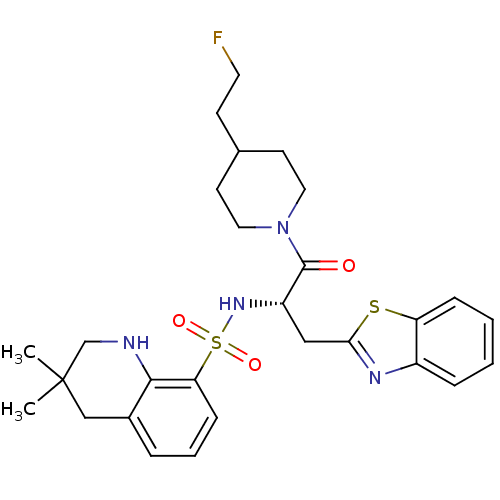 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of human thrombin (in vitro) | Bioorg Med Chem Lett 9: 1317-22 (1999) BindingDB Entry DOI: 10.7270/Q2ZW1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50077058 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090222 (CHEMBL39086 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090244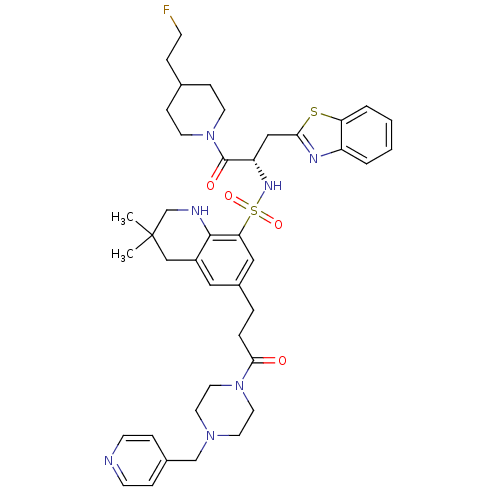 (3,3-Dimethyl-6-[3-oxo-3-(4-pyridin-4-ylmethyl-pipe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090222 (CHEMBL39086 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090247 (6-[3-(4-Acetyl-piperazin-1-yl)-3-oxo-propyl]-3,3-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090250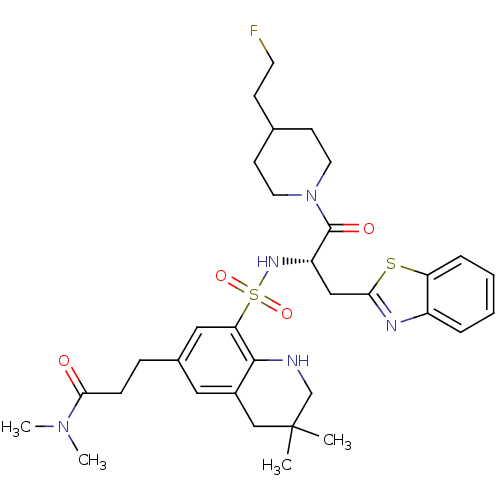 (3-(8-{(S)-1-Benzothiazol-2-ylmethyl-2-[4-(2-fluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50077049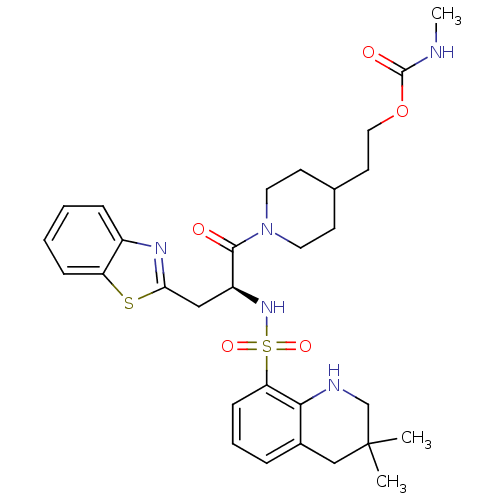 (CHEMBL24186 | Methyl-carbamic acid 2-{1-[(S)-3-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of human thrombin (in vitro) | Bioorg Med Chem Lett 9: 1317-22 (1999) BindingDB Entry DOI: 10.7270/Q2ZW1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090243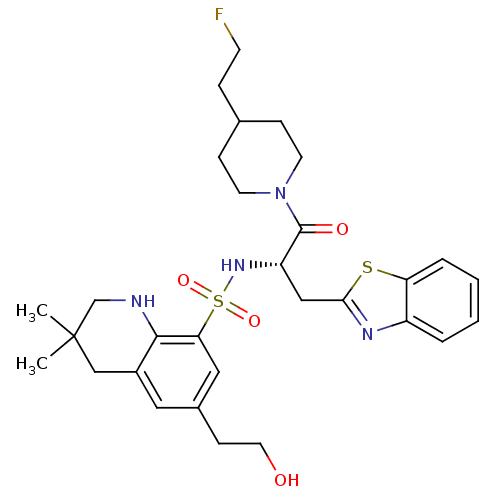 (6-(2-Hydroxy-ethyl)-3,3-dimethyl-1,2,3,4-tetrahydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090246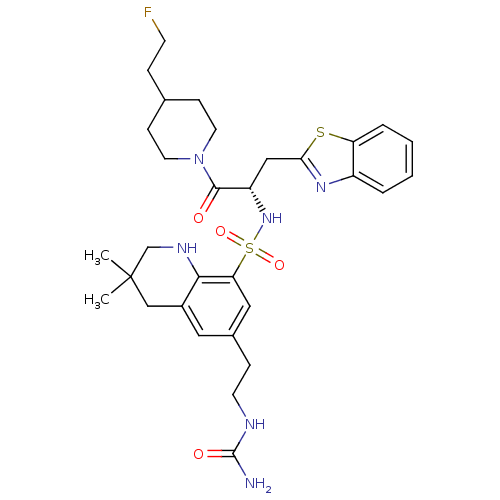 (3,3-Dimethyl-6-(2-ureido-ethyl)-1,2,3,4-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50077053 ((2-{1-[(S)-3-Benzothiazol-2-yl-2-(3,3-dimethyl-1,2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of human thrombin (in vitro) | Bioorg Med Chem Lett 9: 1317-22 (1999) BindingDB Entry DOI: 10.7270/Q2ZW1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50077044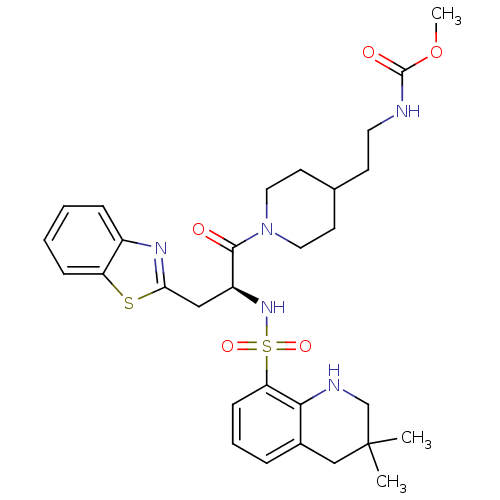 ((2-{1-[(S)-3-Benzothiazol-2-yl-2-(3,3-dimethyl-1,2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of human thrombin (in vitro) | Bioorg Med Chem Lett 9: 1317-22 (1999) BindingDB Entry DOI: 10.7270/Q2ZW1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090238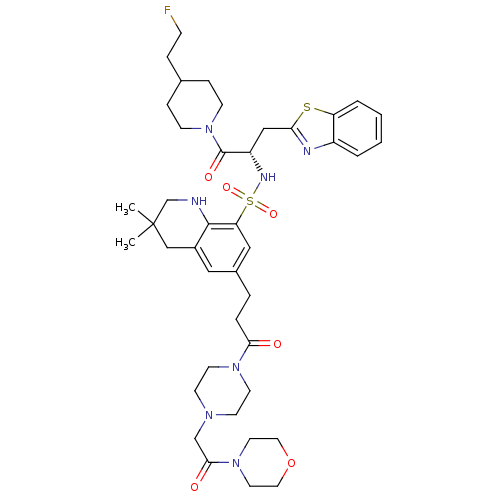 (3,3-Dimethyl-6-{3-[4-(2-morpholin-4-yl-2-oxo-ethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090245 (6-Hydroxymethyl-3,3-dimethyl-1,2,3,4-tetrahydro-qu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50077043 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of human thrombin (in vitro) | Bioorg Med Chem Lett 9: 1317-22 (1999) BindingDB Entry DOI: 10.7270/Q2ZW1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090248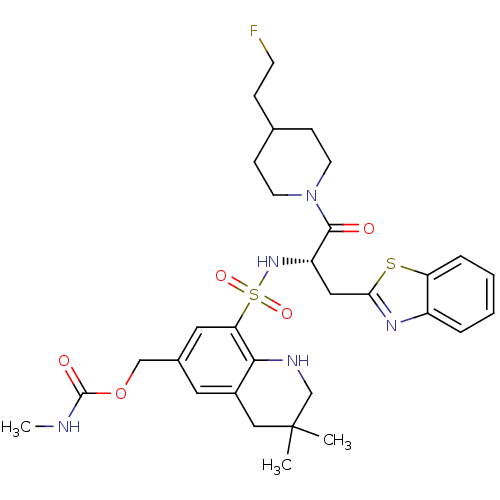 (CHEMBL43397 | Methyl-carbamic acid 8-{(S)-1-benzot...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50077056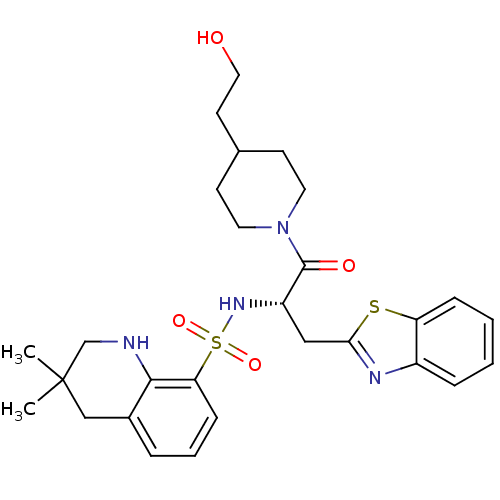 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of human thrombin (in vitro) | Bioorg Med Chem Lett 9: 1317-22 (1999) BindingDB Entry DOI: 10.7270/Q2ZW1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090241 (3-(8-{(S)-1-Benzothiazol-2-ylmethyl-2-[4-(2-fluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50077042 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of human thrombin (in vitro) | Bioorg Med Chem Lett 9: 1317-22 (1999) BindingDB Entry DOI: 10.7270/Q2ZW1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090242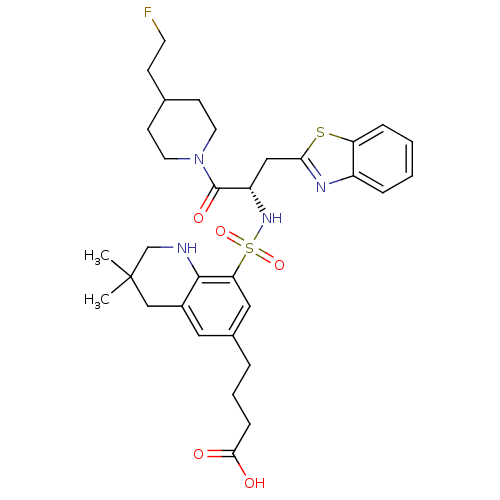 (4-(8-{(S)-1-Benzothiazol-2-ylmethyl-2-[4-(2-fluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090226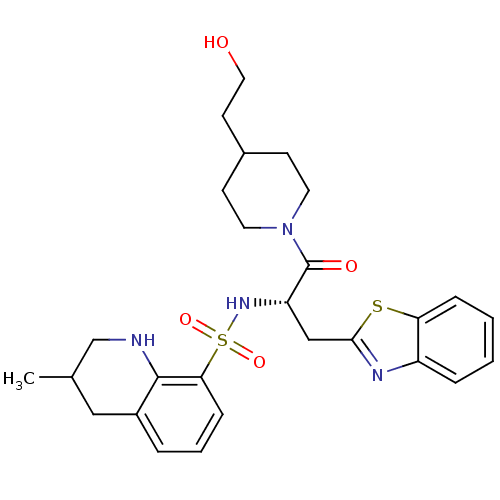 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090226 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090253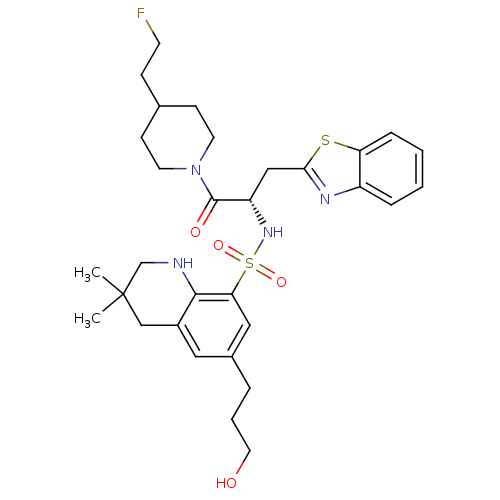 (6-(3-Hydroxy-propyl)-3,3-dimethyl-1,2,3,4-tetrahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50077052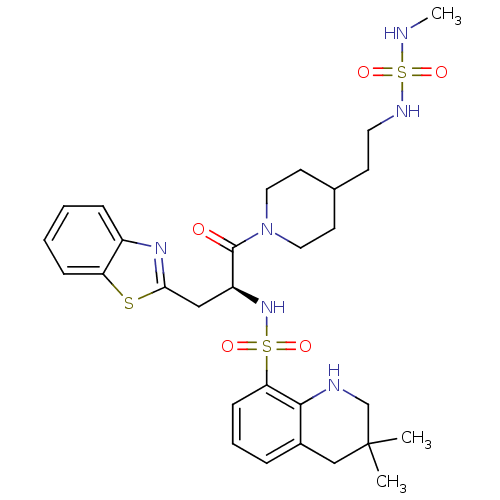 (1N-(2-{1-[3-benzo[d][1,3]thiazol-2-yl-2-(3,3-dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of human thrombin (in vitro) | Bioorg Med Chem Lett 9: 1317-22 (1999) BindingDB Entry DOI: 10.7270/Q2ZW1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090251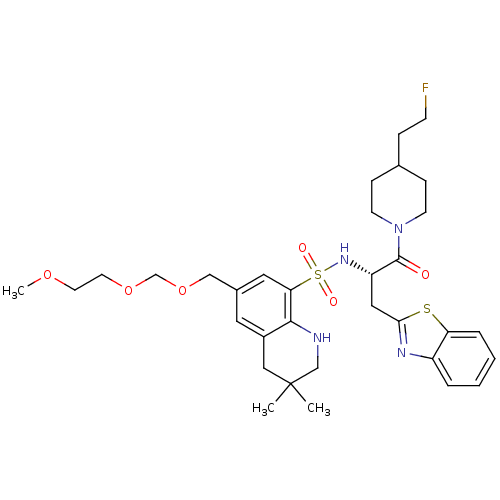 (6-(2-Methoxy-ethoxymethoxymethyl)-3,3-dimethyl-1,2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50366643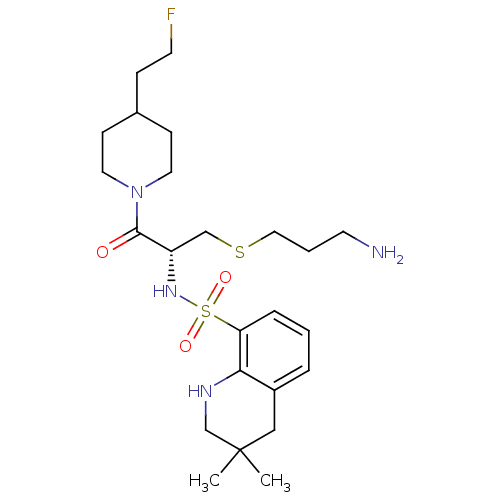 (CHEMBL1907778) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50077046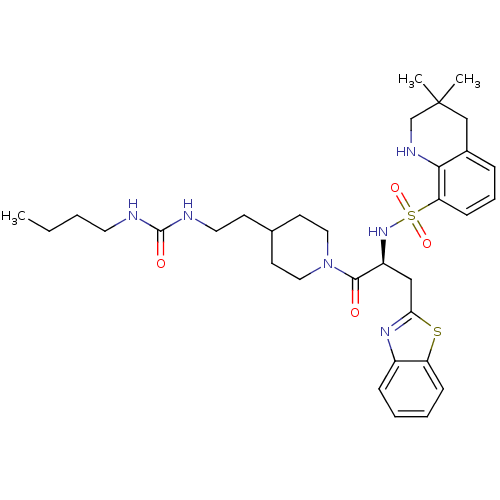 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of human thrombin (in vitro) | Bioorg Med Chem Lett 9: 1317-22 (1999) BindingDB Entry DOI: 10.7270/Q2ZW1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50077050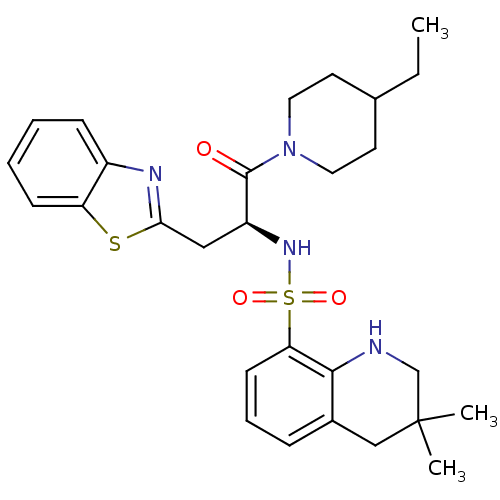 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of human thrombin (in vitro) | Bioorg Med Chem Lett 9: 1317-22 (1999) BindingDB Entry DOI: 10.7270/Q2ZW1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090231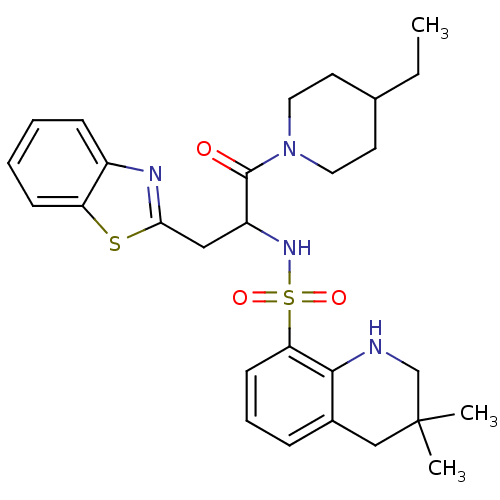 (CHEMBL39375 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50366642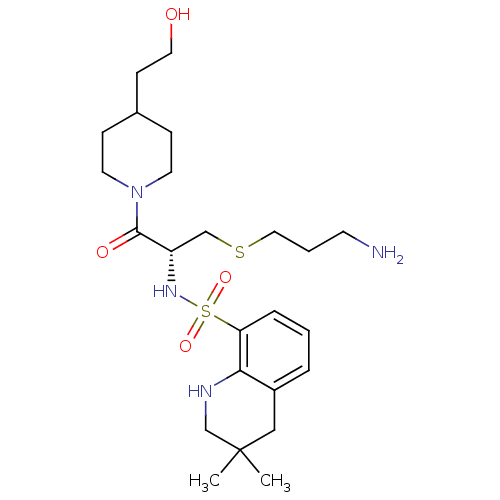 (CHEMBL1907779) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50077047 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of human thrombin (in vitro) | Bioorg Med Chem Lett 9: 1317-22 (1999) BindingDB Entry DOI: 10.7270/Q2ZW1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090240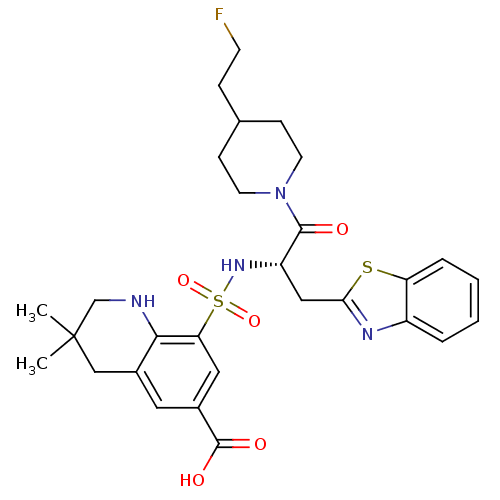 (8-{(S)-1-Benzothiazol-2-ylmethyl-2-[4-(2-fluoro-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 359 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090236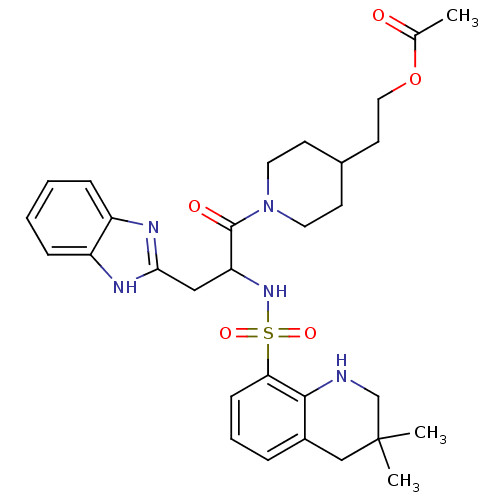 (CHEMBL418248 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 452 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090239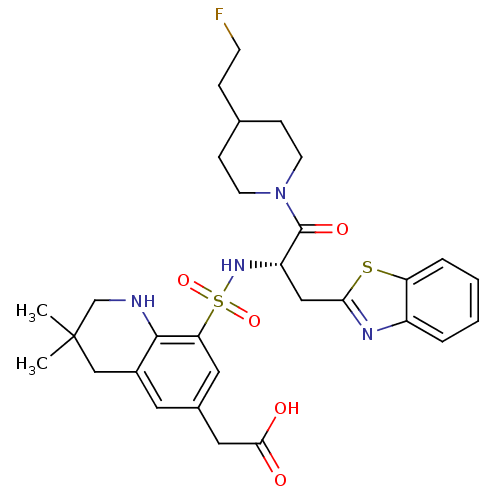 ((8-{(S)-1-Benzothiazol-2-ylmethyl-2-[4-(2-fluoro-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50077051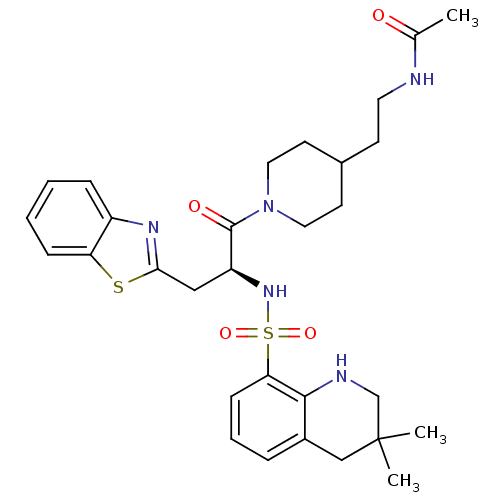 (CHEMBL28272 | N-(2-{1-[(S)-3-Benzothiazol-2-yl-2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 654 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of human thrombin (in vitro) | Bioorg Med Chem Lett 9: 1317-22 (1999) BindingDB Entry DOI: 10.7270/Q2ZW1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090252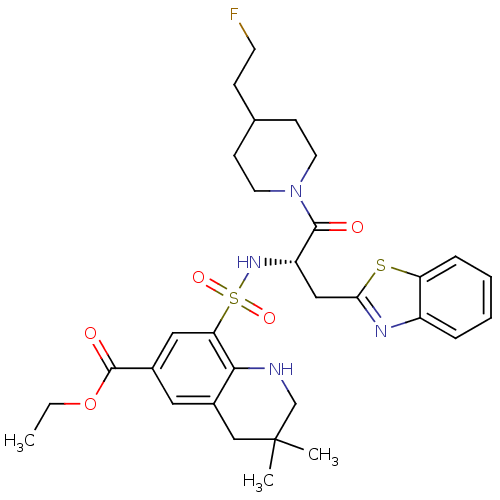 (8-{(S)-1-Benzothiazol-2-ylmethyl-2-[4-(2-fluoro-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 723 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090235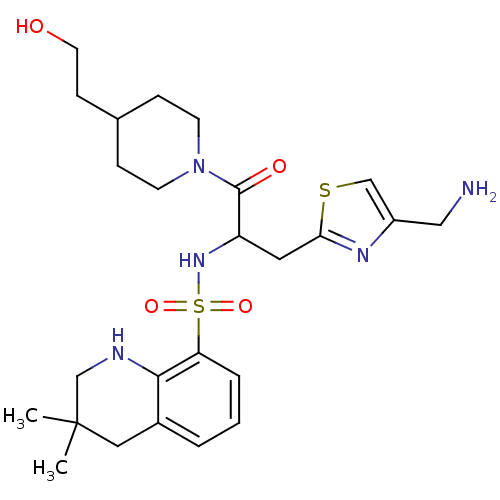 (CHEMBL38907 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090237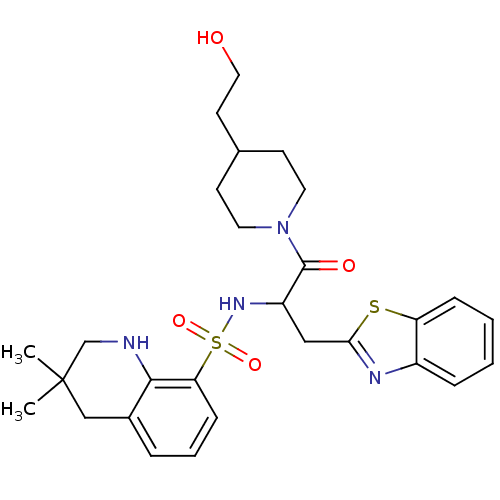 (CHEMBL43074 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090220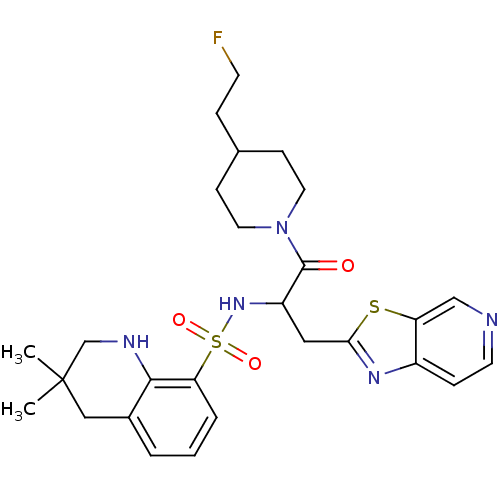 (CHEMBL289623 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50077054 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of human thrombin (in vitro) | Bioorg Med Chem Lett 9: 1317-22 (1999) BindingDB Entry DOI: 10.7270/Q2ZW1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50077048 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of human thrombin (in vitro) | Bioorg Med Chem Lett 9: 1317-22 (1999) BindingDB Entry DOI: 10.7270/Q2ZW1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50366643 (CHEMBL1907778) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Trypsin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090234 (CHEMBL40904 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50366642 (CHEMBL1907779) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Trypsin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090230 (CHEMBL38617 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090224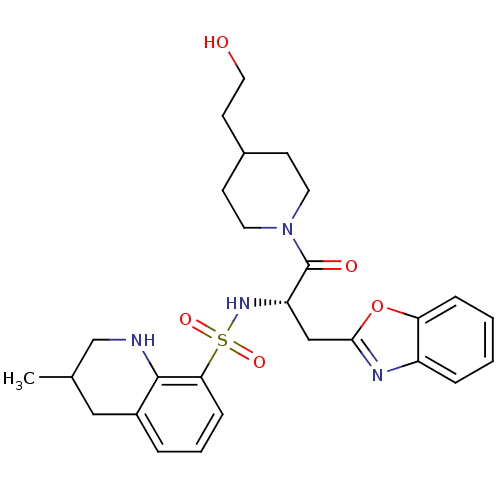 (CHEMBL289493 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 677 total ) | Next | Last >> |