| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Melanocortin receptor 5 |
|---|
| Ligand | BDBM50119367 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_100392 (CHEMBL708684) |
|---|
| EC50 | 127±n/a nM |
|---|
| Citation |  Sebhat, IK; Martin, WJ; Ye, Z; Barakat, K; Mosley, RT; Johnston, DB; Bakshi, R; Palucki, B; Weinberg, DH; MacNeil, T; Kalyani, RN; Tang, R; Stearns, RA; Miller, RR; Tamvakopoulos, C; Strack, AM; McGowan, E; Cashen, DE; Drisko, JE; Hom, GJ; Howard, AD; MacIntyre, DE; van der Ploeg, LH; Patchett, AA; Nargund, RP Design and pharmacology of N-[(3R)-1,2,3,4-tetrahydroisoquinolinium- 3-ylcarbonyl]-(1R)-1-(4-chlorobenzyl)- 2-[4-cyclohexyl-4-(1H-1,2,4-triazol- 1-ylmethyl)piperidin-1-yl]-2-oxoethylamine (1), a potent, selective, melanocortin subtype-4 receptor agonist. J Med Chem45:4589-93 (2002) [PubMed] Sebhat, IK; Martin, WJ; Ye, Z; Barakat, K; Mosley, RT; Johnston, DB; Bakshi, R; Palucki, B; Weinberg, DH; MacNeil, T; Kalyani, RN; Tang, R; Stearns, RA; Miller, RR; Tamvakopoulos, C; Strack, AM; McGowan, E; Cashen, DE; Drisko, JE; Hom, GJ; Howard, AD; MacIntyre, DE; van der Ploeg, LH; Patchett, AA; Nargund, RP Design and pharmacology of N-[(3R)-1,2,3,4-tetrahydroisoquinolinium- 3-ylcarbonyl]-(1R)-1-(4-chlorobenzyl)- 2-[4-cyclohexyl-4-(1H-1,2,4-triazol- 1-ylmethyl)piperidin-1-yl]-2-oxoethylamine (1), a potent, selective, melanocortin subtype-4 receptor agonist. J Med Chem45:4589-93 (2002) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Melanocortin receptor 5 |
|---|
| Name: | Melanocortin receptor 5 |
|---|
| Synonyms: | MC-2 | MC5-R | MC5R | MC5R_HUMAN | Melanocortin MC5 | Melanocortin receptor (M4 and M5) | Melanocortin receptor 5 | Melanocortin receptor 5 (MC5R) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 36612.92 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P33032 |
|---|
| Residue: | 325 |
|---|
| Sequence: | MNSSFHLHFLDLNLNATEGNLSGPNVKNKSSPCEDMGIAVEVFLTLGVISLLENILVIGA
IVKNKNLHSPMYFFVCSLAVADMLVSMSSAWETITIYLLNNKHLVIADAFVRHIDNVFDS
MICISVVASMCSLLAIAVDRYVTIFYALRYHHIMTARRSGAIIAGIWAFCTGCGIVFILY
SESTYVILCLISMFFAMLFLLVSLYIHMFLLARTHVKRIAALPGASSARQRTSMQGAVTV
TMLLGVFTVCWAPFFLHLTLMLSCPQNLYCSRFMSHFNMYLILIMCNSVMDPLIYAFRSQ
EMRKTFKEIICCRGFRIACSFPRRD
|
|
|
|---|
| BDBM50119367 |
|---|
| n/a |
|---|
| Name | BDBM50119367 |
|---|
| Synonyms: | (3R)-N-[(2R)-3-(4-chlorophenyl)-1-{4-cyclohexyl-4-[(1-methyl-1H-1,2,3,4-tetrazol-5-yl)methyl]piperidin-1-yl}-1-oxopropan-2-yl]-1,2,3,4-tetrahydroisoquinoline-3-carboxamide | 1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid {1-(4-chloro-benzyl)-2-[4-cyclohexyl-4-(1-methyl-1H-tetrazol-5-ylmethyl)-piperidin-1-yl]-2-oxo-ethyl}-amide | CHEMBL140154 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C33H42ClN7O2 |
|---|
| Mol. Mass. | 604.185 |
|---|
| SMILES | Cn1nnnc1CC1(CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1)C1CCCCC1 |
|---|
| Structure | 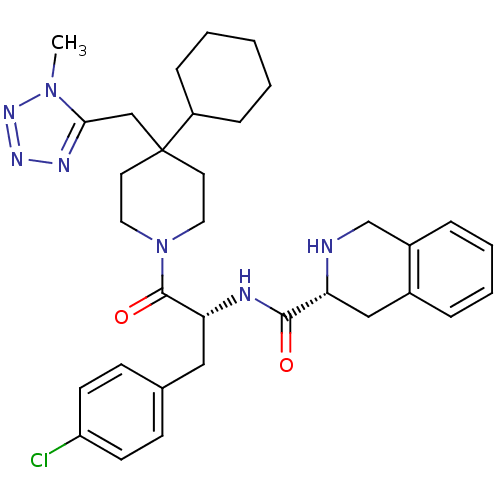
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Sebhat, IK; Martin, WJ; Ye, Z; Barakat, K; Mosley, RT; Johnston, DB; Bakshi, R; Palucki, B; Weinberg, DH; MacNeil, T; Kalyani, RN; Tang, R; Stearns, RA; Miller, RR; Tamvakopoulos, C; Strack, AM; McGowan, E; Cashen, DE; Drisko, JE; Hom, GJ; Howard, AD; MacIntyre, DE; van der Ploeg, LH; Patchett, AA; Nargund, RP Design and pharmacology of N-[(3R)-1,2,3,4-tetrahydroisoquinolinium- 3-ylcarbonyl]-(1R)-1-(4-chlorobenzyl)- 2-[4-cyclohexyl-4-(1H-1,2,4-triazol- 1-ylmethyl)piperidin-1-yl]-2-oxoethylamine (1), a potent, selective, melanocortin subtype-4 receptor agonist. J Med Chem45:4589-93 (2002) [PubMed]
Sebhat, IK; Martin, WJ; Ye, Z; Barakat, K; Mosley, RT; Johnston, DB; Bakshi, R; Palucki, B; Weinberg, DH; MacNeil, T; Kalyani, RN; Tang, R; Stearns, RA; Miller, RR; Tamvakopoulos, C; Strack, AM; McGowan, E; Cashen, DE; Drisko, JE; Hom, GJ; Howard, AD; MacIntyre, DE; van der Ploeg, LH; Patchett, AA; Nargund, RP Design and pharmacology of N-[(3R)-1,2,3,4-tetrahydroisoquinolinium- 3-ylcarbonyl]-(1R)-1-(4-chlorobenzyl)- 2-[4-cyclohexyl-4-(1H-1,2,4-triazol- 1-ylmethyl)piperidin-1-yl]-2-oxoethylamine (1), a potent, selective, melanocortin subtype-4 receptor agonist. J Med Chem45:4589-93 (2002) [PubMed]