

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119367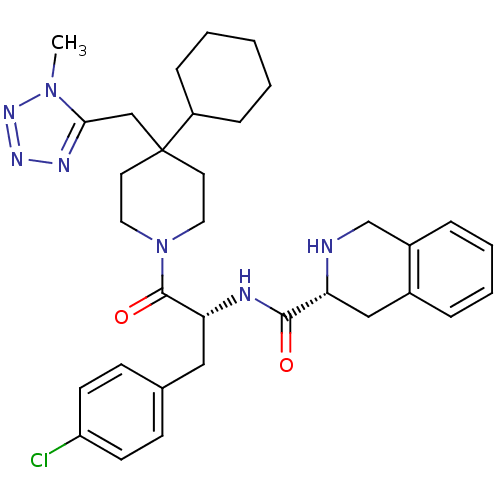 ((3R)-N-[(2R)-3-(4-chlorophenyl)-1-{4-cyclohexyl-4-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for binding affinity against Melanocortin-4 receptor by displacing [125I]-NDP-alpha-MSH radioligand expressed in CHO cells | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50098391 ((S)-4-(2-(azetidin-2-yl)ethoxy)-7-chloro-2-oxo-N-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of binding of the compound to GnRH receptor in human | J Med Chem 44: 917-22 (2001) BindingDB Entry DOI: 10.7270/Q2H70F3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119371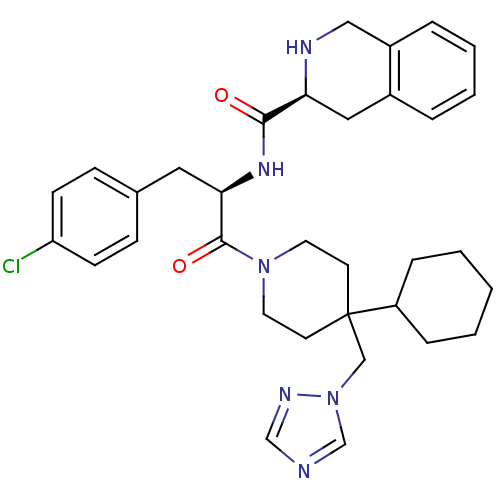 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for binding affinity against Melanocortin-4 receptor by displacing [125I]-NDP-alpha-MSH radioligand expressed in CHO cells | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50098391 ((S)-4-(2-(azetidin-2-yl)ethoxy)-7-chloro-2-oxo-N-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of gonadotropin-releasing hormone receptor in rhesus monkey | J Med Chem 44: 917-22 (2001) BindingDB Entry DOI: 10.7270/Q2H70F3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Rattus norvegicus) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for binding affinity against rat Melanocortin-4 receptor (rMC4R) by displacing [125I]NDP-alpha-MSH radioligand expressed in CHO cells | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50098391 ((S)-4-(2-(azetidin-2-yl)ethoxy)-7-chloro-2-oxo-N-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Functional antagonism at the human GnRH receptor (PI turnover) | J Med Chem 44: 917-22 (2001) BindingDB Entry DOI: 10.7270/Q2H70F3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for binding affinity against human Melanocortin-4 receptor (hMC4R) by displacing [125I]-NDP-alpha-MSH radioligand expressed in CHO cells | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for Functional Activity at Melanocortin-4 receptor as effective concentration at 50% maximum CMP accumulation | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119366 ((R)-N-((R)-3-(4-chlorophenyl)-1-(4-cyclohexyl-4-((...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for binding affinity against Melanocortin-4 receptor by displacing [125I]-NDP-alpha-MSH radioligand expressed in CHO cells | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50098391 ((S)-4-(2-(azetidin-2-yl)ethoxy)-7-chloro-2-oxo-N-(...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of binding of the compound to GnRH receptor in rat | J Med Chem 44: 917-22 (2001) BindingDB Entry DOI: 10.7270/Q2H70F3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50098391 ((S)-4-(2-(azetidin-2-yl)ethoxy)-7-chloro-2-oxo-N-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Functional antagonism at the rhesus monkey gonadotropin-releasing hormone receptor | J Med Chem 44: 917-22 (2001) BindingDB Entry DOI: 10.7270/Q2H70F3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50098391 ((S)-4-(2-(azetidin-2-yl)ethoxy)-7-chloro-2-oxo-N-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of binding of the compound to gonadotropin-releasing hormone receptor in dog | J Med Chem 44: 917-22 (2001) BindingDB Entry DOI: 10.7270/Q2H70F3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50098391 ((S)-4-(2-(azetidin-2-yl)ethoxy)-7-chloro-2-oxo-N-(...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Functional antagonism at GnRH receptor in rat (PI turnover) | J Med Chem 44: 917-22 (2001) BindingDB Entry DOI: 10.7270/Q2H70F3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119373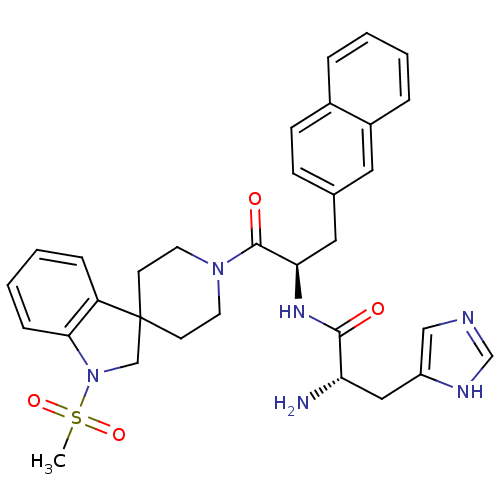 ((2S)-2-amino-3-(1H-imidazol-4-yl)-N-[(2R)-1-{1-met...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for Functional Activity at Melanocortin-4 receptor as effective concentration at 50% maximum CMP accumulation | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119369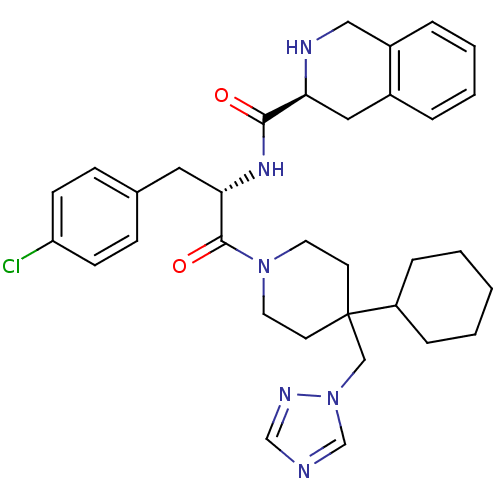 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for Functional Activity at Melanocortin-4 receptor as effective concentration at 50% maximum CMP accumulation | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119372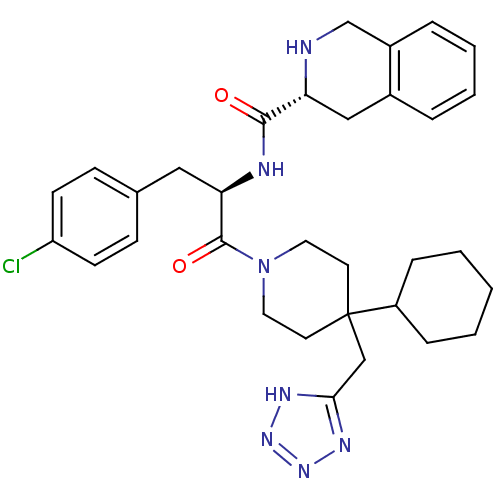 ((R)-N-((R)-1-(4-((1H-tetrazol-5-yl)methyl)-4-cyclo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for Functional Activity at Melanocortin-4 receptor as effective concentration at 50% maximum CMP accumulation | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 326 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for binding affinity against human melanocortin 5 (hMC5R) receptor by displacing [125I]-NDP-alpha-MSH radioligand expressed in CHO cells | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50098391 ((S)-4-(2-(azetidin-2-yl)ethoxy)-7-chloro-2-oxo-N-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Functional antagonism at gonadotropin-releasing hormone receptor in dog (PI turnover) | J Med Chem 44: 917-22 (2001) BindingDB Entry DOI: 10.7270/Q2H70F3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 761 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for binding affinity against human Melanocortin-3 receptor (hMC3R) by displacing [125I]NDP-alpha-MSH radioligand expressed in CHO cells | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Rattus norvegicus) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for binding affinity against rat Melanocortin-5 receptor (rMC5R) by displacing [125I]NDP-alpha-MSH radioligand expressed in CHO cells | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Rattus norvegicus) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for binding affinity against rat Melanocortin-3 receptor (rMC3R) by displacing [125I]-NDP-alpha-MSH radioligand expressed in CHO cells | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for binding affinity against human melanocortin 1 (hMC1R) receptor by displacing [125I]-NDP-alpha-MSH radioligand expressed in CHO cells | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119370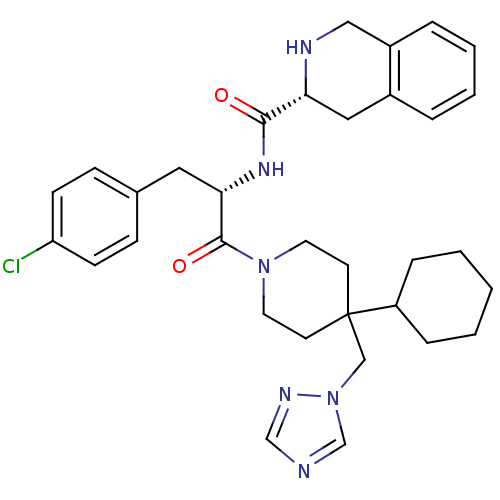 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for Functional Activity at Melanocortin-4 receptor as effective concentration at 50% maximum CMP accumulation | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 2.49E+3 | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Functional activity as concentration of the compound at 50% maximum cAMP accumulation in human melanocortin 1 (MC3R) receptor | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 2.49E+3 | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for Functional Activity at MC3R as effective concentration at 50% maximum CMP accumulation | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for Functional Activity at Melanocortin-4 receptor as effective concentration at 50% maximum CMP accumulation | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119365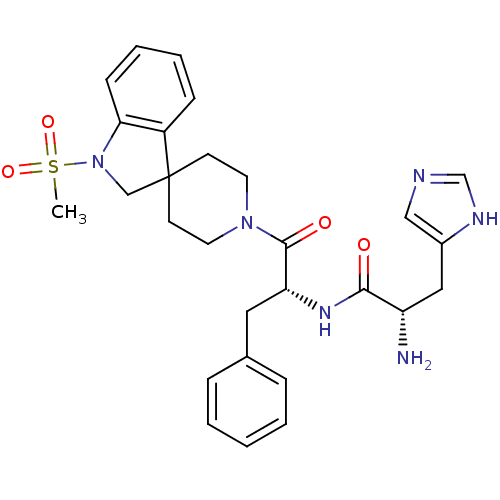 ((2S)-2-amino-3-(1H-imidazol-4-yl)-N-[(2R)-1-{1-met...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for Functional Activity at Melanocortin-4 receptor as effective concentration at 50% maximum CMP accumulation | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119366 ((R)-N-((R)-3-(4-chlorophenyl)-1-(4-cyclohexyl-4-((...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for Functional Activity at Melanocortin-4 receptor as effective concentration at 50% maximum CMP accumulation | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50119367 ((3R)-N-[(2R)-3-(4-chlorophenyl)-1-{4-cyclohexyl-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for Functional Activity at MC3R as effective concentration at 50% maximum CMP accumulation | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119367 ((3R)-N-[(2R)-3-(4-chlorophenyl)-1-{4-cyclohexyl-4-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for Functional Activity at Melanocortin-4 receptor as effective concentration at 50% maximum CMP accumulation | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50119366 ((R)-N-((R)-3-(4-chlorophenyl)-1-(4-cyclohexyl-4-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for Functional Activity at MC5R as effective concentration at 50% maximum CMP accumulation | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119371 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for Functional Activity at Melanocortin-4 receptor as effective concentration at 50% maximum CMP accumulation | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50119367 ((3R)-N-[(2R)-3-(4-chlorophenyl)-1-{4-cyclohexyl-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 127 | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for Functional Activity at MC5R as effective concentration at 50% maximum CMP accumulation | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Rattus norvegicus) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Functional activity as concentration of the compound at 50% maximum cAMP accumulation in rat Melanocortin-4 receptor (rMC4R) | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Functional activity as concentration of the compound at 50% maximum cAMP accumulation in human Melanocortin-4 receptor | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50119371 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 271 | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for Functional Activity at MC5R as effective concentration at 50% maximum CMP accumulation | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Rattus norvegicus) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Functional activity as concentration of the compound at 50% maximum cAMP accumulation in rat Melanocortin-3 receptor (rMC3R) | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50119372 ((R)-N-((R)-1-(4-((1H-tetrazol-5-yl)methyl)-4-cyclo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 771 | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for Functional Activity at Melanocortin-4 receptor as effective concentration at 50% maximum CMP accumulation | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 2.85E+3 | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Functional activity as concentration at 50% maximum cAMP accumulation in human melanocortin 1 (MC1R) receptor | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 737 | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Functional activity as concentration at 50% maximum cAMP accumulation in human melanocortin 1 (MC5R) receptor | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 736 | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Evaluated for Functional Activity at MC5R as effective concentration at 50% maximum CMP accumulation | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Rattus norvegicus) | BDBM50119368 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Functional activity as concentration of the compound at 50% maximum cAMP accumulation in rat Melanocortin-5 receptor (rMC5R) | J Med Chem 45: 4589-93 (2002) BindingDB Entry DOI: 10.7270/Q2GT5MH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||