| Reaction Details |
|---|
 | Report a problem with these data |
| Target | UDP-glucuronosyltransferase 1A1 |
|---|
| Ligand | BDBM50576021 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2126229 (CHEMBL4835574) |
|---|
| IC50 | 1100±n/a nM |
|---|
| Citation |  Konteatis, Z; Travins, J; Gross, S; Marjon, K; Barnett, A; Mandley, E; Nicolay, B; Nagaraja, R; Chen, Y; Sun, Y; Liu, Z; Yu, J; Ye, Z; Jiang, F; Wei, W; Fang, C; Gao, Y; Kalev, P; Hyer, ML; DeLaBarre, B; Jin, L; Padyana, AK; Dang, L; Murtie, J; Biller, SA; Sui, Z; Marks, KM Discovery of AG-270, a First-in-Class Oral MAT2A Inhibitor for the Treatment of Tumors with Homozygous J Med Chem64:4430-4449 (2021) [PubMed] Article Konteatis, Z; Travins, J; Gross, S; Marjon, K; Barnett, A; Mandley, E; Nicolay, B; Nagaraja, R; Chen, Y; Sun, Y; Liu, Z; Yu, J; Ye, Z; Jiang, F; Wei, W; Fang, C; Gao, Y; Kalev, P; Hyer, ML; DeLaBarre, B; Jin, L; Padyana, AK; Dang, L; Murtie, J; Biller, SA; Sui, Z; Marks, KM Discovery of AG-270, a First-in-Class Oral MAT2A Inhibitor for the Treatment of Tumors with Homozygous J Med Chem64:4430-4449 (2021) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| UDP-glucuronosyltransferase 1A1 |
|---|
| Name: | UDP-glucuronosyltransferase 1A1 |
|---|
| Synonyms: | Bilirubin-specific UDPGT isozyme 1 | GNT1 | UD11_HUMAN | UDP-glucuronosyltransferase 1-1 | UDP-glucuronosyltransferase 1-A | UDP-glucuronosyltransferase 1A1 | UDPGT 1-1 | UGT-1A | UGT1 | UGT1*1 | UGT1-01 | UGT1.1 | UGT1A | UGT1A1 | Uridine-5'-diphosphoglucuronosyltransferase 1A1 | hUG-BR1 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 59604.34 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P22309 |
|---|
| Residue: | 533 |
|---|
| Sequence: | MAVESQGGRPLVLGLLLCVLGPVVSHAGKILLIPVDGSHWLSMLGAIQQLQQRGHEIVVL
APDASLYIRDGAFYTLKTYPVPFQREDVKESFVSLGHNVFENDSFLQRVIKTYKKIKKDS
AMLLSGCSHLLHNKELMASLAESSFDVMLTDPFLPCSPIVAQYLSLPTVFFLHALPCSLE
FEATQCPNPFSYVPRPLSSHSDHMTFLQRVKNMLIAFSQNFLCDVVYSPYATLASEFLQR
EVTVQDLLSSASVWLFRSDFVKDYPRPIMPNMVFVGGINCLHQNPLSQEFEAYINASGEH
GIVVFSLGSMVSEIPEKKAMAIADALGKIPQTVLWRYTGTRPSNLANNTILVKWLPQNDL
LGHPMTRAFITHAGSHGVYESICNGVPMVMMPLFGDQMDNAKRMETKGAGVTLNVLEMTS
EDLENALKAVINDKSYKENIMRLSSLHKDRPVEPLDLAVFWVEFVMRHKGAPHLRPAAHD
LTWYQYHSLDVIGFLLAVVLTVAFITFKCCAYGYRKCLGKKGRVKKAHKSKTH
|
|
|
|---|
| BDBM50576021 |
|---|
| n/a |
|---|
| Name | BDBM50576021 |
|---|
| Synonyms: | CHEMBL4573938 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C30H27N5O2 |
|---|
| Mol. Mass. | 489.5677 |
|---|
| SMILES | COc1ccc(cc1)-c1c(Nc2ccccn2)[nH]c2c(c(nn2c1=O)-c1ccccc1)C1=CCCCC1 |t:36| |
|---|
| Structure | 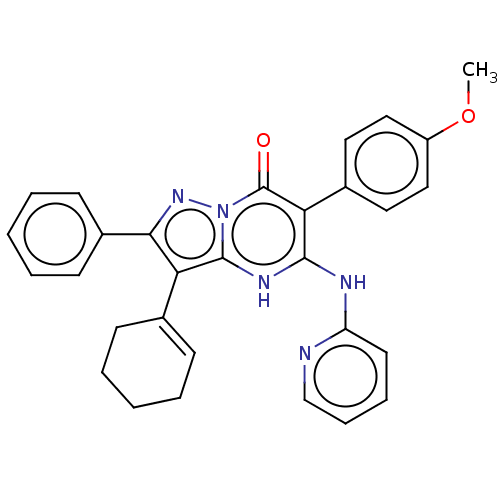
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Konteatis, Z; Travins, J; Gross, S; Marjon, K; Barnett, A; Mandley, E; Nicolay, B; Nagaraja, R; Chen, Y; Sun, Y; Liu, Z; Yu, J; Ye, Z; Jiang, F; Wei, W; Fang, C; Gao, Y; Kalev, P; Hyer, ML; DeLaBarre, B; Jin, L; Padyana, AK; Dang, L; Murtie, J; Biller, SA; Sui, Z; Marks, KM Discovery of AG-270, a First-in-Class Oral MAT2A Inhibitor for the Treatment of Tumors with Homozygous J Med Chem64:4430-4449 (2021) [PubMed] Article
Konteatis, Z; Travins, J; Gross, S; Marjon, K; Barnett, A; Mandley, E; Nicolay, B; Nagaraja, R; Chen, Y; Sun, Y; Liu, Z; Yu, J; Ye, Z; Jiang, F; Wei, W; Fang, C; Gao, Y; Kalev, P; Hyer, ML; DeLaBarre, B; Jin, L; Padyana, AK; Dang, L; Murtie, J; Biller, SA; Sui, Z; Marks, KM Discovery of AG-270, a First-in-Class Oral MAT2A Inhibitor for the Treatment of Tumors with Homozygous J Med Chem64:4430-4449 (2021) [PubMed] Article