 Found 17212 hits with Last Name = 'yu' and Initial = 'j'
Found 17212 hits with Last Name = 'yu' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50430798
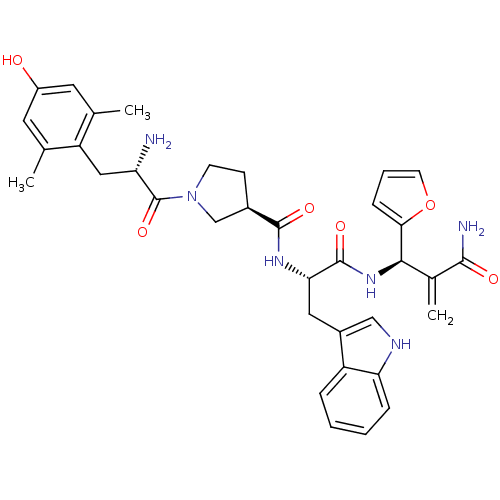
(CHEMBL2335120)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CC[C@H](C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccco1 |r| Show InChI InChI=1S/C35H40N6O6/c1-19-13-24(42)14-20(2)26(19)16-27(36)35(46)41-11-10-22(18-41)33(44)39-29(15-23-17-38-28-8-5-4-7-25(23)28)34(45)40-31(21(3)32(37)43)30-9-6-12-47-30/h4-9,12-14,17,22,27,29,31,38,42H,3,10-11,15-16,18,36H2,1-2H3,(H2,37,43)(H,39,44)(H,40,45)/t22-,27+,29+,31-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... |
J Med Chem 56: 3102-14 (2013)
Article DOI: 10.1021/jm400195y
BindingDB Entry DOI: 10.7270/Q2KS6SW8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50214974
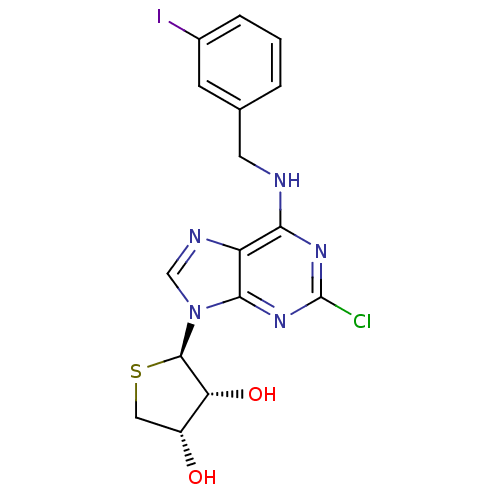
((2R,3R,4S)-2-(2-chloro-6-(3-iodobenzylamino)-9H-pu...)Show SMILES O[C@@H]1CS[C@H]([C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C16H15ClIN5O2S/c17-16-21-13(19-5-8-2-1-3-9(18)4-8)11-14(22-16)23(7-20-11)15-12(25)10(24)6-26-15/h1-4,7,10,12,15,24-25H,5-6H2,(H,19,21,22)/t10-,12-,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00483 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University
Curated by ChEMBL
| Assay Description
Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARdelta LBD by TR-FRET assay |
J Med Chem 60: 7459-7475 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00805
BindingDB Entry DOI: 10.7270/Q2XK8HQH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50430801
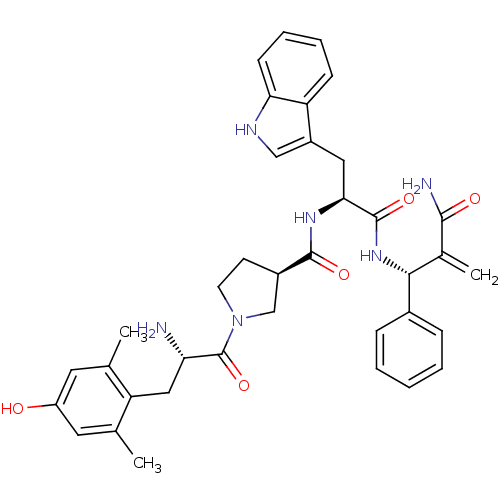
(CHEMBL2334776)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CC[C@H](C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccccc1 |r| Show InChI InChI=1S/C37H42N6O5/c1-21-15-27(44)16-22(2)29(21)18-30(38)37(48)43-14-13-25(20-43)35(46)41-32(17-26-19-40-31-12-8-7-11-28(26)31)36(47)42-33(23(3)34(39)45)24-9-5-4-6-10-24/h4-12,15-16,19,25,30,32-33,40,44H,3,13-14,17-18,20,38H2,1-2H3,(H2,39,45)(H,41,46)(H,42,47)/t25-,30+,32+,33-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00986 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... |
J Med Chem 56: 3102-14 (2013)
Article DOI: 10.1021/jm400195y
BindingDB Entry DOI: 10.7270/Q2KS6SW8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50214981
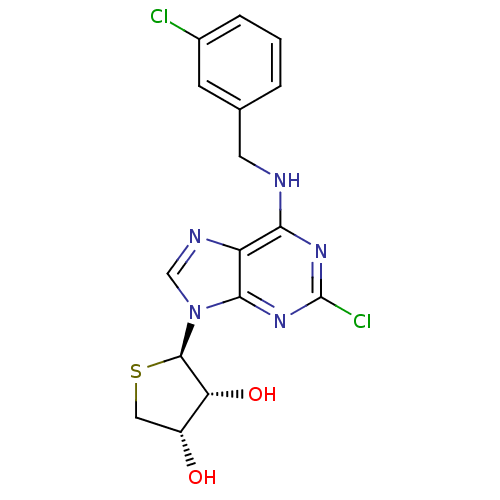
((2R,3R,4S)-2-(2-chloro-6-(3-chlorobenzylamino)-9H-...)Show SMILES O[C@@H]1CS[C@H]([C@@H]1O)n1cnc2c(NCc3cccc(Cl)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C16H15Cl2N5O2S/c17-9-3-1-2-8(4-9)5-19-13-11-14(22-16(18)21-13)23(7-20-11)15-12(25)10(24)6-26-15/h1-4,7,10,12,15,24-25H,5-6H2,(H,19,21,22)/t10-,12-,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University
Curated by ChEMBL
| Assay Description
Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARdelta LBD by TR-FRET assay |
J Med Chem 60: 7459-7475 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00805
BindingDB Entry DOI: 10.7270/Q2XK8HQH |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18793
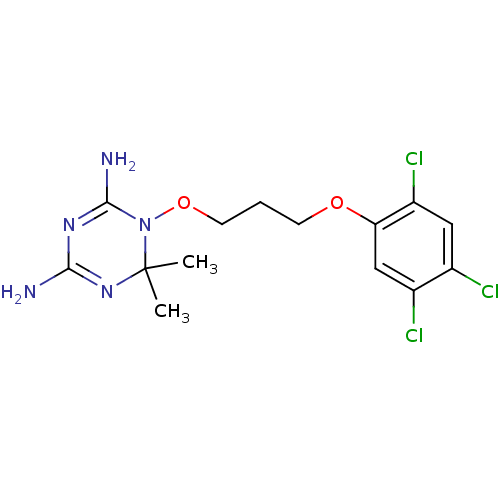
(6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]...)Show SMILES CC1(C)N=C(N)N=C(N)N1OCCCOc1cc(Cl)c(Cl)cc1Cl |t:3,6| Show InChI InChI=1S/C14H18Cl3N5O2/c1-14(2)21-12(18)20-13(19)22(14)24-5-3-4-23-11-7-9(16)8(15)6-10(11)17/h6-7H,3-5H2,1-2H3,(H4,18,19,20,21) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 0.0110 | -62.5 | 0.570 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University
| Assay Description
The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... |
Nat Struct Biol 10: 257-65 (2003)
Article DOI: 10.1038/nsb921
BindingDB Entry DOI: 10.7270/Q2HH6HBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50430802
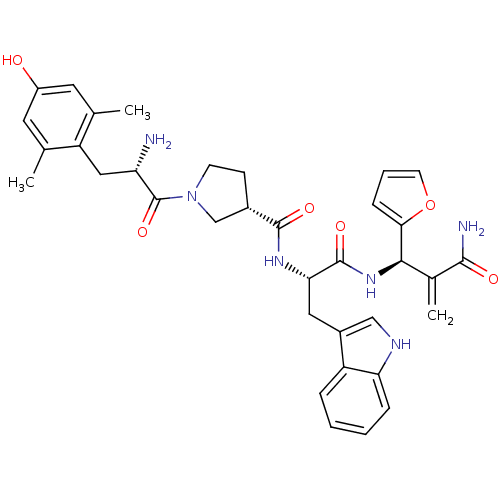
(CHEMBL2334775)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CC[C@@H](C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccco1 |r| Show InChI InChI=1S/C35H40N6O6/c1-19-13-24(42)14-20(2)26(19)16-27(36)35(46)41-11-10-22(18-41)33(44)39-29(15-23-17-38-28-8-5-4-7-25(23)28)34(45)40-31(21(3)32(37)43)30-9-6-12-47-30/h4-9,12-14,17,22,27,29,31,38,42H,3,10-11,15-16,18,36H2,1-2H3,(H2,37,43)(H,39,44)(H,40,45)/t22-,27-,29-,31+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... |
J Med Chem 56: 3102-14 (2013)
Article DOI: 10.1021/jm400195y
BindingDB Entry DOI: 10.7270/Q2KS6SW8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50430803
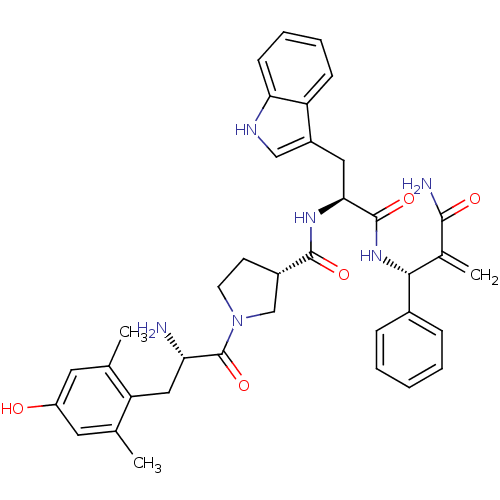
(CHEMBL2334774)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CC[C@@H](C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccccc1 |r| Show InChI InChI=1S/C37H42N6O5/c1-21-15-27(44)16-22(2)29(21)18-30(38)37(48)43-14-13-25(20-43)35(46)41-32(17-26-19-40-31-12-8-7-11-28(26)31)36(47)42-33(23(3)34(39)45)24-9-5-4-6-10-24/h4-12,15-16,19,25,30,32-33,40,44H,3,13-14,17-18,20,38H2,1-2H3,(H2,39,45)(H,41,46)(H,42,47)/t25-,30-,32-,33+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... |
J Med Chem 56: 3102-14 (2013)
Article DOI: 10.1021/jm400195y
BindingDB Entry DOI: 10.7270/Q2KS6SW8 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18793

(6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]...)Show SMILES CC1(C)N=C(N)N=C(N)N1OCCCOc1cc(Cl)c(Cl)cc1Cl |t:3,6| Show InChI InChI=1S/C14H18Cl3N5O2/c1-14(2)21-12(18)20-13(19)22(14)24-5-3-4-23-11-7-9(16)8(15)6-10(11)17/h6-7H,3-5H2,1-2H3,(H4,18,19,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 0.0200 | -61.1 | 2.30 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University
| Assay Description
The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... |
Nat Struct Biol 10: 257-65 (2003)
Article DOI: 10.1038/nsb921
BindingDB Entry DOI: 10.7270/Q2HH6HBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50430799
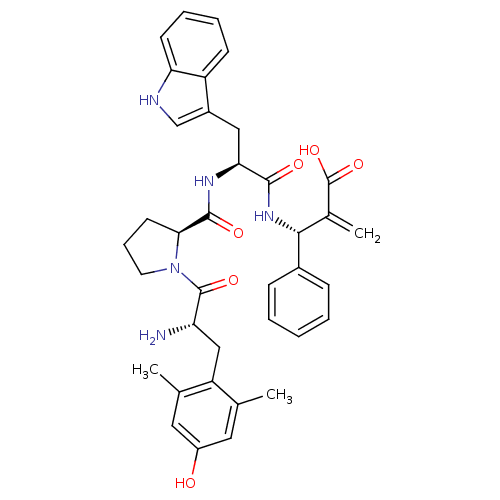
(CHEMBL2334772)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C37H41N5O6/c1-21-16-26(43)17-22(2)28(21)19-29(38)36(46)42-15-9-14-32(42)35(45)40-31(18-25-20-39-30-13-8-7-12-27(25)30)34(44)41-33(23(3)37(47)48)24-10-5-4-6-11-24/h4-8,10-13,16-17,20,29,31-33,39,43H,3,9,14-15,18-19,38H2,1-2H3,(H,40,45)(H,41,44)(H,47,48)/t29-,31-,32-,33+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0322 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... |
J Med Chem 56: 3102-14 (2013)
Article DOI: 10.1021/jm400195y
BindingDB Entry DOI: 10.7270/Q2KS6SW8 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase [N51I,C59R,S108N,I164L]
(Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18793

(6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]...)Show SMILES CC1(C)N=C(N)N=C(N)N1OCCCOc1cc(Cl)c(Cl)cc1Cl |t:3,6| Show InChI InChI=1S/C14H18Cl3N5O2/c1-14(2)21-12(18)20-13(19)22(14)24-5-3-4-23-11-7-9(16)8(15)6-10(11)17/h6-7H,3-5H2,1-2H3,(H4,18,19,20,21) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 0.0370 | -59.5 | 18 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University
| Assay Description
The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... |
Nat Struct Biol 10: 257-65 (2003)
Article DOI: 10.1038/nsb921
BindingDB Entry DOI: 10.7270/Q2HH6HBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50430800
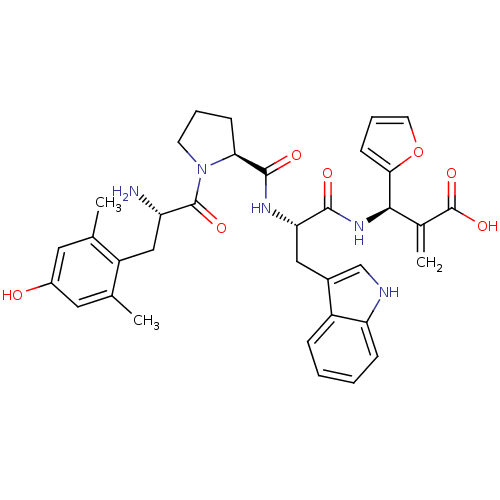
(CHEMBL2334773)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(O)=O)c1ccco1 |r| Show InChI InChI=1S/C35H39N5O7/c1-19-14-23(41)15-20(2)25(19)17-26(36)34(44)40-12-6-10-29(40)33(43)38-28(16-22-18-37-27-9-5-4-8-24(22)27)32(42)39-31(21(3)35(45)46)30-11-7-13-47-30/h4-5,7-9,11,13-15,18,26,28-29,31,37,41H,3,6,10,12,16-17,36H2,1-2H3,(H,38,43)(H,39,42)(H,45,46)/t26-,28-,29-,31+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... |
J Med Chem 56: 3102-14 (2013)
Article DOI: 10.1021/jm400195y
BindingDB Entry DOI: 10.7270/Q2KS6SW8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM28661
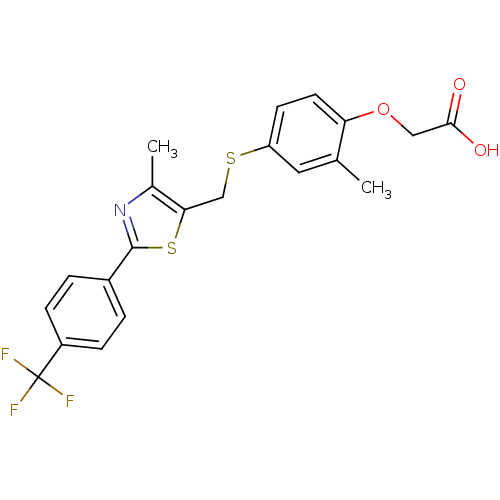
(2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...)Show SMILES Cc1nc(sc1CSc1ccc(OCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO3S2/c1-12-9-16(7-8-17(12)28-10-19(26)27)29-11-18-13(2)25-20(30-18)14-3-5-15(6-4-14)21(22,23)24/h3-9H,10-11H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University
Curated by ChEMBL
| Assay Description
Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARdelta LBD by TR-FRET assay |
J Med Chem 60: 7459-7475 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00805
BindingDB Entry DOI: 10.7270/Q2XK8HQH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085048
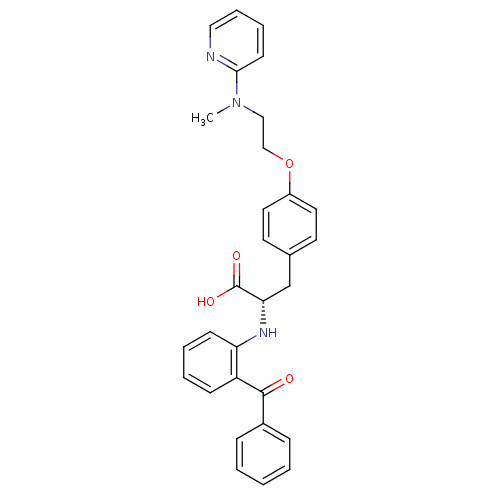
((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(methyl-pyri...)Show SMILES CN(CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)c1ccccn1 Show InChI InChI=1S/C30H29N3O4/c1-33(28-13-7-8-18-31-28)19-20-37-24-16-14-22(15-17-24)21-27(30(35)36)32-26-12-6-5-11-25(26)29(34)23-9-3-2-4-10-23/h2-18,27,32H,19-21H2,1H3,(H,35,36)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0497 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University
Curated by ChEMBL
| Assay Description
Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARgamma LBD by TR-FRET assay |
J Med Chem 60: 7459-7475 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00805
BindingDB Entry DOI: 10.7270/Q2XK8HQH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50099491
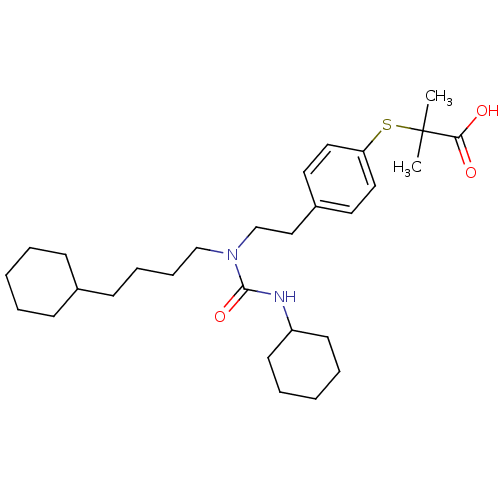
(2-(4-(2-(3-cyclohexyl-1-(4-cyclohexylbutyl)ureido)...)Show SMILES CC(C)(Sc1ccc(CCN(CCCCC2CCCCC2)C(=O)NC2CCCCC2)cc1)C(O)=O Show InChI InChI=1S/C29H46N2O3S/c1-29(2,27(32)33)35-26-18-16-24(17-19-26)20-22-31(28(34)30-25-14-7-4-8-15-25)21-10-9-13-23-11-5-3-6-12-23/h16-19,23,25H,3-15,20-22H2,1-2H3,(H,30,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0541 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University
Curated by ChEMBL
| Assay Description
Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARalpha LBD by TR-FRET assay |
J Med Chem 60: 7459-7475 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00805
BindingDB Entry DOI: 10.7270/Q2XK8HQH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50512416

(CHEMBL4447162)Show SMILES Nc1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCCCCCn3cc(CCCCN4CCC(CC4)c4ccc(cc4)-c4cc(cc5cc(ccc45)-c4ccc(cc4)C(F)(F)F)C(O)=O)nn3)c3ccc(=N)c(c3oc2c1S(O)(=O)=O)S(O)(=O)=O |(41.72,-13.14,;41.86,-14.67,;40.6,-15.57,;40.74,-17.1,;42.13,-17.74,;42.28,-19.27,;41.03,-20.17,;41.18,-21.7,;39.93,-22.59,;38.53,-21.95,;38.38,-20.42,;39.63,-19.53,;39.47,-17.99,;37.94,-17.87,;39.87,-16.5,;37.28,-22.84,;37.42,-24.37,;35.88,-22.2,;34.62,-23.09,;33.22,-22.45,;31.97,-23.35,;30.57,-22.71,;29.31,-23.6,;27.91,-22.96,;26.66,-23.85,;25.2,-23.36,;24.28,-24.6,;22.74,-24.59,;21.98,-23.25,;20.44,-23.23,;19.69,-21.89,;18.15,-21.87,;17.36,-23.2,;15.83,-23.18,;15.07,-21.84,;15.84,-20.51,;17.39,-20.53,;13.54,-21.84,;12.76,-23.17,;11.22,-23.15,;10.46,-21.81,;11.23,-20.49,;12.77,-20.49,;8.92,-21.8,;8.16,-20.45,;6.61,-20.45,;5.83,-21.78,;6.59,-23.12,;5.82,-24.44,;6.58,-25.78,;8.12,-25.8,;8.9,-24.47,;8.14,-23.13,;5.79,-27.1,;4.25,-27.09,;3.47,-28.41,;4.23,-29.76,;5.78,-29.76,;6.55,-28.44,;3.45,-31.08,;1.91,-31.07,;4.21,-32.42,;2.67,-32.41,;5.84,-19.11,;4.3,-19.1,;6.62,-17.78,;25.17,-25.85,;26.64,-25.39,;43.68,-19.91,;43.82,-21.43,;45.21,-22.07,;46.46,-21.19,;47.86,-21.84,;46.33,-19.66,;44.93,-19.02,;44.8,-17.49,;43.39,-16.85,;43.26,-15.32,;44.51,-14.44,;45.91,-15.09,;43.74,-13.1,;45.28,-13.09,;47.58,-18.77,;48.98,-19.41,;46.8,-17.43,;48.35,-17.43,)| Show InChI InChI=1S/C62H58F3N7O12S2/c63-62(64,65)44-16-12-37(13-17-44)40-14-18-46-42(31-40)32-43(60(74)75)34-50(46)39-10-8-36(9-11-39)38-24-29-71(30-25-38)27-6-3-7-45-35-72(70-69-45)28-5-2-1-4-26-68-59(73)41-15-19-47(51(33-41)61(76)77)54-48-20-22-52(66)57(85(78,79)80)55(48)84-56-49(54)21-23-53(67)58(56)86(81,82)83/h8-23,31-35,38,66H,1-7,24-30,67H2,(H,68,73)(H,74,75)(H,76,77)(H,78,79,80)(H,81,82,83) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Inhibition of human P2Y14R |
J Med Chem 63: 9563-9589 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00745
BindingDB Entry DOI: 10.7270/Q20R9SZP |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50122294
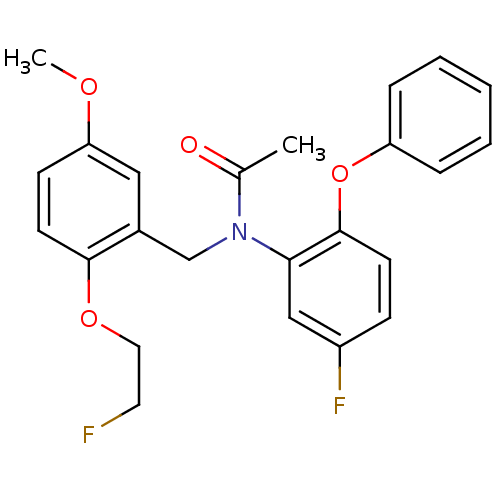
(CHEMBL292092 | N-(5-fluoro-2-phenoxyphenyl)-N-(2-(...)Show SMILES COc1ccc(OCCF)c(CN(C(C)=O)c2cc(F)ccc2Oc2ccccc2)c1 Show InChI InChI=1S/C24H23F2NO4/c1-17(28)27(16-18-14-21(29-2)9-11-23(18)30-13-12-25)22-15-19(26)8-10-24(22)31-20-6-4-3-5-7-20/h3-11,14-15H,12-13,16H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences
Curated by ChEMBL
| Assay Description
Displacement of [11C]DAA1106 from PBR receptor in rat brain membrane |
Bioorg Med Chem Lett 19: 1707-10 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.093
BindingDB Entry DOI: 10.7270/Q2C82B6C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328391
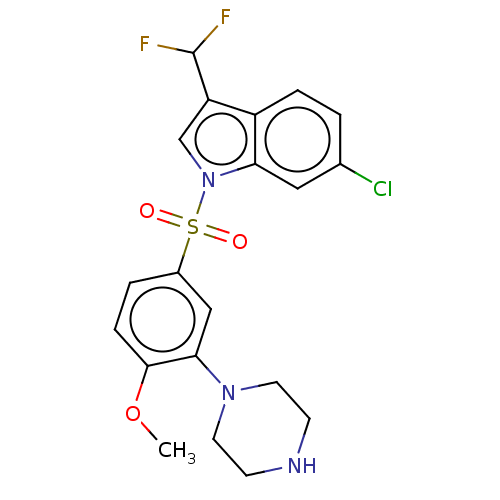
(6-chloro-3-(difluoromethyl)-1-((4-methoxy-3-(piper...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C(F)F)c2ccc(Cl)cc12 Show InChI InChI=1S/C20H20ClF2N3O3S/c1-29-19-5-3-14(11-18(19)25-8-6-24-7-9-25)30(27,28)26-12-16(20(22)23)15-4-2-13(21)10-17(15)26/h2-5,10-12,20,24H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50297306
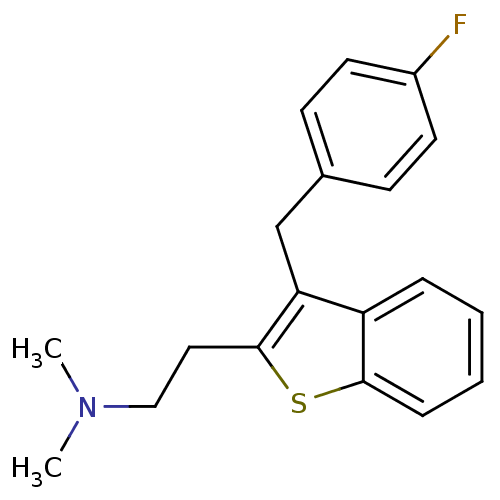
(CHEMBL540982 | {2-[3-(4-Fluoro-benzyl)-benzo[b]thi...)Show InChI InChI=1S/C19H20FNS/c1-21(2)12-11-19-17(13-14-7-9-15(20)10-8-14)16-5-3-4-6-18(16)22-19/h3-10H,11-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in HEK293 Flp-In cells by liquid scintillation counting |
J Med Chem 52: 5307-10 (2009)
Article DOI: 10.1021/jm900933k
BindingDB Entry DOI: 10.7270/Q2057G0S |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(BOVINE) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit the dopamine-stimulated adenylate-cyclase activity in dispersed cells of bovine parathyroid gland |
J Med Chem 33: 521-6 (1990)
BindingDB Entry DOI: 10.7270/Q22J69VZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50297304
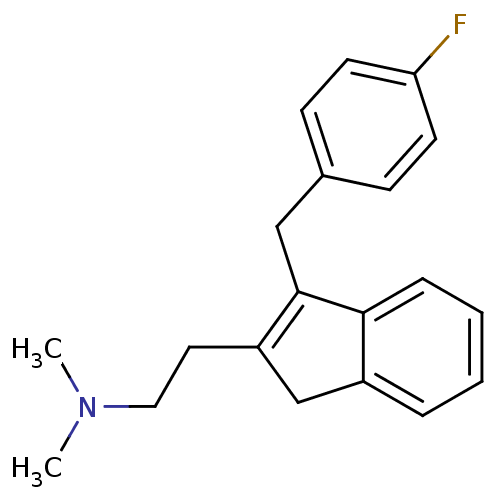
(CHEMBL560741 | {2-[3-(4-Fluoro-benzyl)-1H-inden-2-...)Show InChI InChI=1S/C20H22FN/c1-22(2)12-11-17-14-16-5-3-4-6-19(16)20(17)13-15-7-9-18(21)10-8-15/h3-10H,11-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in HEK293 Flp-In cells by liquid scintillation counting |
J Med Chem 52: 5307-10 (2009)
Article DOI: 10.1021/jm900933k
BindingDB Entry DOI: 10.7270/Q2057G0S |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit [3H]-SCH- 23390 binding to Dopamine receptor D1 of canine striatal membranes |
J Med Chem 33: 521-6 (1990)
BindingDB Entry DOI: 10.7270/Q22J69VZ |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(BOVINE) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit [3H]-SCH- 23390 binding to Dopamine receptor D1 of canine striatal membranes |
J Med Chem 33: 521-6 (1990)
BindingDB Entry DOI: 10.7270/Q22J69VZ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50180197
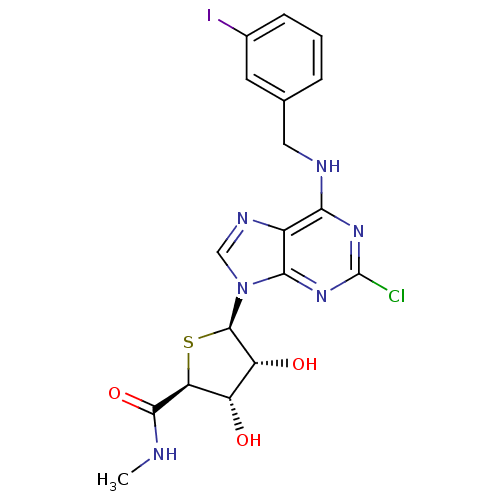
((2S,3S,4R,5R)-5-(2-chloro-6-(3-iodobenzylamino)-9H...)Show SMILES CNC(=O)[C@H]1S[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C18H18ClIN6O3S/c1-21-16(29)13-11(27)12(28)17(30-13)26-7-23-10-14(24-18(19)25-15(10)26)22-6-8-3-2-4-9(20)5-8/h2-5,7,11-13,17,27-28H,6H2,1H3,(H,21,29)(H,22,24,25)/t11-,12+,13-,17+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University
Curated by ChEMBL
| Assay Description
Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARdelta LBD by TR-FRET assay |
J Med Chem 60: 7459-7475 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00805
BindingDB Entry DOI: 10.7270/Q2XK8HQH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50430806
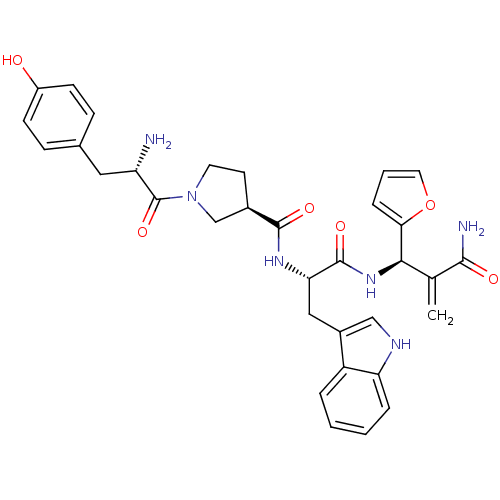
(CHEMBL2334771)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CC[C@H](C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccco1 |r| Show InChI InChI=1S/C33H36N6O6/c1-19(30(35)41)29(28-7-4-14-45-28)38-32(43)27(16-22-17-36-26-6-3-2-5-24(22)26)37-31(42)21-12-13-39(18-21)33(44)25(34)15-20-8-10-23(40)11-9-20/h2-11,14,17,21,25,27,29,36,40H,1,12-13,15-16,18,34H2,(H2,35,41)(H,37,42)(H,38,43)/t21-,25+,27+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... |
J Med Chem 56: 3102-14 (2013)
Article DOI: 10.1021/jm400195y
BindingDB Entry DOI: 10.7270/Q2KS6SW8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50304633
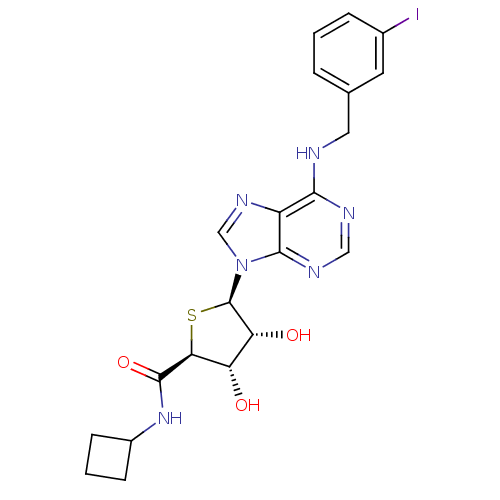
(9-(5'-Cyclobutylaminocarbonyl-4'-thio-beta-D-ribof...)Show SMILES O[C@H]1[C@@H](O)[C@@H](S[C@@H]1C(=O)NC1CCC1)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C21H23IN6O3S/c22-12-4-1-3-11(7-12)8-23-18-14-19(25-9-24-18)28(10-26-14)21-16(30)15(29)17(32-21)20(31)27-13-5-2-6-13/h1,3-4,7,9-10,13,15-17,21,29-30H,2,5-6,8H2,(H,27,31)(H,23,24,25)/t15-,16+,17-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University
Curated by ChEMBL
| Assay Description
Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARgamma LBD by TR-FRET assay |
J Med Chem 60: 7459-7475 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00805
BindingDB Entry DOI: 10.7270/Q2XK8HQH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50592784
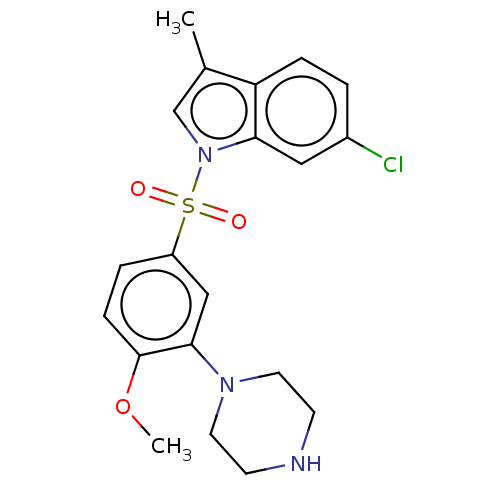
(CHEMBL5177311)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C)c2ccc(Cl)cc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18512

(5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...)Show InChI InChI=1S/C12H13ClN4/c1-2-9-10(11(14)17-12(15)16-9)7-3-5-8(13)6-4-7/h3-6H,2H2,1H3,(H4,14,15,16,17) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | -55.4 | 80 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University
| Assay Description
The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... |
Nat Struct Biol 10: 257-65 (2003)
Article DOI: 10.1038/nsb921
BindingDB Entry DOI: 10.7270/Q2HH6HBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50401333
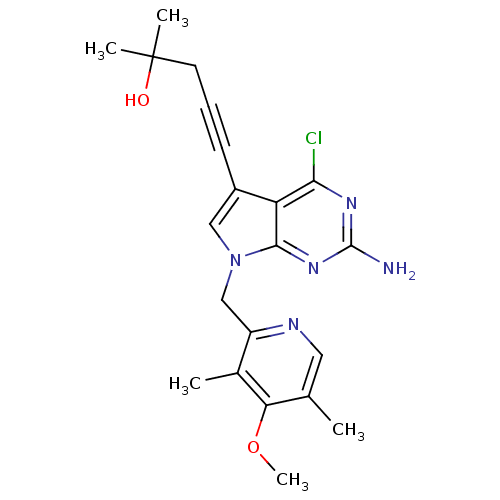
(CHEMBL1230584)Show SMILES COc1c(C)cnc(Cn2cc(C#CCC(C)(C)O)c3c(Cl)nc(N)nc23)c1C Show InChI InChI=1S/C21H24ClN5O2/c1-12-9-24-15(13(2)17(12)29-5)11-27-10-14(7-6-8-21(3,4)28)16-18(22)25-20(23)26-19(16)27/h9-10,28H,8,11H2,1-5H3,(H2,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Binding affinity at recombinant Hsp90alpha incubated for 16 hrs by fluorescence polarization competition assay |
J Med Chem 55: 7786-95 (2012)
Article DOI: 10.1021/jm300810x
BindingDB Entry DOI: 10.7270/Q2V125Z3 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50266889

(CHEMBL513922 | N-benzyl-N-ethyl-2-(7-methyl-8-oxo-...)Show SMILES CCN(Cc1ccccc1)C(=O)Cn1c2nc(ncc2n(C)c1=O)-c1ccccc1 Show InChI InChI=1S/C23H23N5O2/c1-3-27(15-17-10-6-4-7-11-17)20(29)16-28-22-19(26(2)23(28)30)14-24-21(25-22)18-12-8-5-9-13-18/h4-14H,3,15-16H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences
Curated by ChEMBL
| Assay Description
Displacement of [11C]PK11195 from PBR receptor in rat brain membrane |
Bioorg Med Chem Lett 19: 1707-10 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.093
BindingDB Entry DOI: 10.7270/Q2C82B6C |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049529
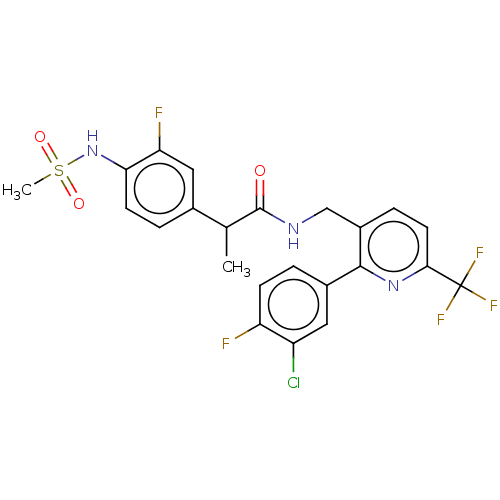
(CHEMBL3317476)Show SMILES CC(C(=O)NCc1ccc(nc1-c1ccc(F)c(Cl)c1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H19ClF5N3O3S/c1-12(13-4-7-19(18(26)10-13)32-36(2,34)35)22(33)30-11-15-5-8-20(23(27,28)29)31-21(15)14-3-6-17(25)16(24)9-14/h3-10,12,32H,11H2,1-2H3,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced channel activation by FLIPR assay |
Bioorg Med Chem Lett 24: 4044-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.072
BindingDB Entry DOI: 10.7270/Q2JH3NTH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328379
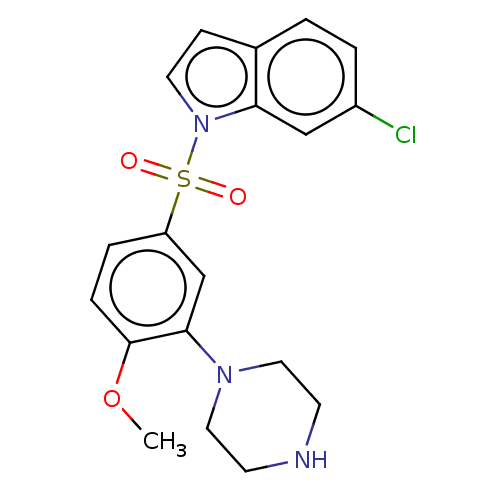
(6-chloro-1-((4-methoxy-3-(piperazin-1-yl)phenyl)su...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1ccc2ccc(Cl)cc12 Show InChI InChI=1S/C19H20ClN3O3S/c1-26-19-5-4-16(13-18(19)22-10-7-21-8-11-22)27(24,25)23-9-6-14-2-3-15(20)12-17(14)23/h2-6,9,12-13,21H,7-8,10-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50395060
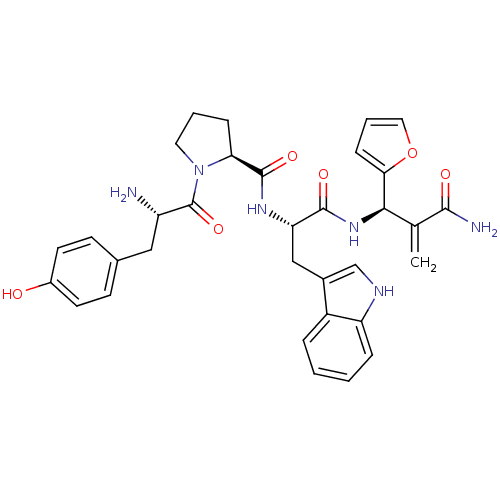
(CHEMBL2163916)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccco1 |r| Show InChI InChI=1S/C33H36N6O6/c1-19(30(35)41)29(28-9-5-15-45-28)38-31(42)26(17-21-18-36-25-7-3-2-6-23(21)25)37-32(43)27-8-4-14-39(27)33(44)24(34)16-20-10-12-22(40)13-11-20/h2-3,5-7,9-13,15,18,24,26-27,29,36,40H,1,4,8,14,16-17,34H2,(H2,35,41)(H,37,43)(H,38,42)/t24-,26-,27-,29+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from FLAG-tagged mu opioid receptor expressed in HEK293 cells after 60 mins by liquid scintillation counter |
J Med Chem 55: 6224-36 (2012)
Article DOI: 10.1021/jm300664y
BindingDB Entry DOI: 10.7270/Q2T154S8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260806
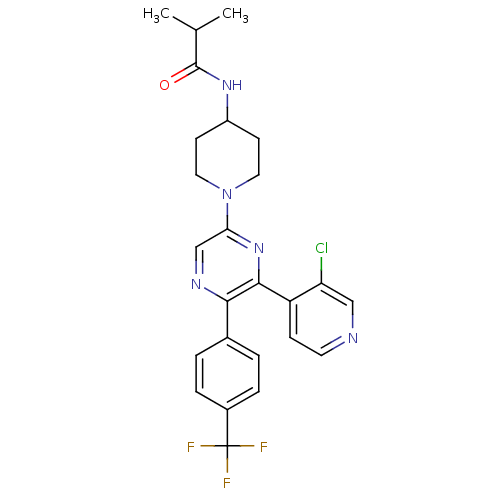
(CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...)Show SMILES CC(C)C(=O)NC1CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccncc1Cl Show InChI InChI=1S/C25H25ClF3N5O/c1-15(2)24(35)32-18-8-11-34(12-9-18)21-14-31-22(16-3-5-17(6-4-16)25(27,28)29)23(33-21)19-7-10-30-13-20(19)26/h3-7,10,13-15,18H,8-9,11-12H2,1-2H3,(H,32,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50252829
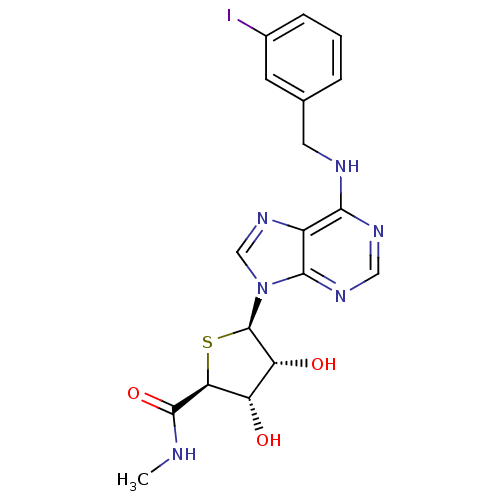
((2S,3S,4R,5R)-5-(6-(3-iodobenzylamino)-9H-purin-9-...)Show SMILES CNC(=O)[C@H]1S[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O3S/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting analysis |
J Med Chem 60: 3422-3437 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00241
BindingDB Entry DOI: 10.7270/Q2J105FT |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50243173
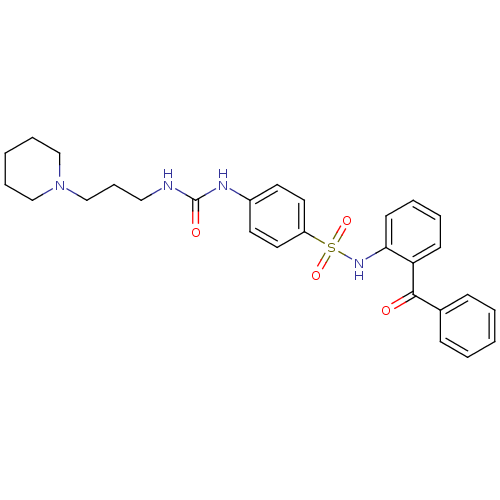
(CHEMBL487445 | N-(2-Benzoyl-phenyl)-4-[3-(3-piperi...)Show SMILES O=C(NCCCN1CCCCC1)Nc1ccc(cc1)S(=O)(=O)Nc1ccccc1C(=O)c1ccccc1 Show InChI InChI=1S/C28H32N4O4S/c33-27(22-10-3-1-4-11-22)25-12-5-6-13-26(25)31-37(35,36)24-16-14-23(15-17-24)30-28(34)29-18-9-21-32-19-7-2-8-20-32/h1,3-6,10-17,31H,2,7-9,18-21H2,(H2,29,30,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human bradykinin B1 receptor |
J Med Chem 51: 3946-52 (2008)
Article DOI: 10.1021/jm800199h
BindingDB Entry DOI: 10.7270/Q2JH3N33 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 17B
(Homo sapiens (Human)) | BDBM50166121
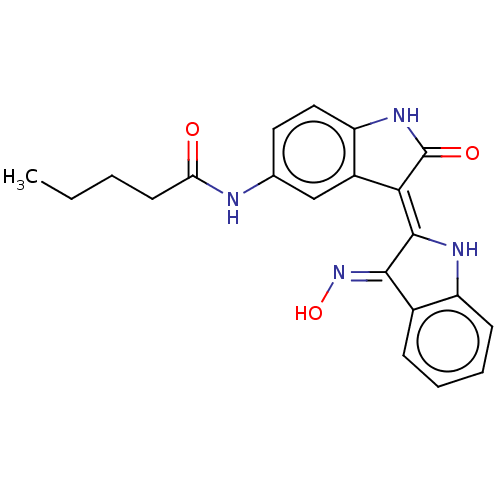
(CHEMBL3797480)Show SMILES CCCCC(=O)Nc1ccc2NC(=O)\C(=C3/Nc4ccccc4/C/3=N\O)c2c1 Show InChI InChI=1S/C21H20N4O3/c1-2-3-8-17(26)22-12-9-10-16-14(11-12)18(21(27)24-16)20-19(25-28)13-6-4-5-7-15(13)23-20/h4-7,9-11,23,28H,2-3,8H2,1H3,(H,22,26)(H,24,27)/b20-18-,25-19+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Competitive inhibition of DRAK2 (unknown origin) using MRLC3 peptide as substrate incubated for 2 hrs by Lineweaver-Burk plot analysis in presence of... |
Bioorg Med Chem Lett 26: 2719-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.111
BindingDB Entry DOI: 10.7270/Q2N29ZTZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50130285
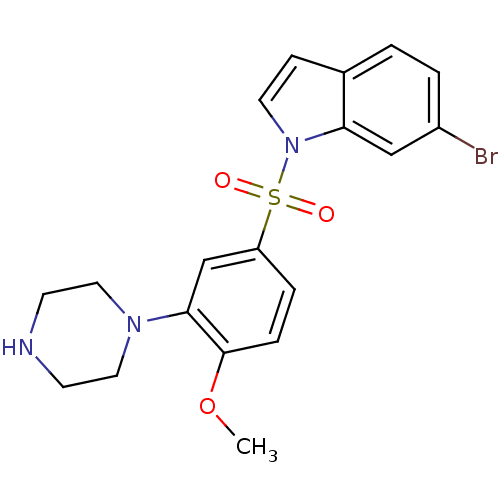
(6-Bromo-1-(4-methoxy-3-piperazin-1-yl-benzenesulfo...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1ccc2ccc(Br)cc12 Show InChI InChI=1S/C19H20BrN3O3S/c1-26-19-5-4-16(13-18(19)22-10-7-21-8-11-22)27(24,25)23-9-6-14-2-3-15(20)12-17(14)23/h2-6,9,12-13,21H,7-8,10-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50592785
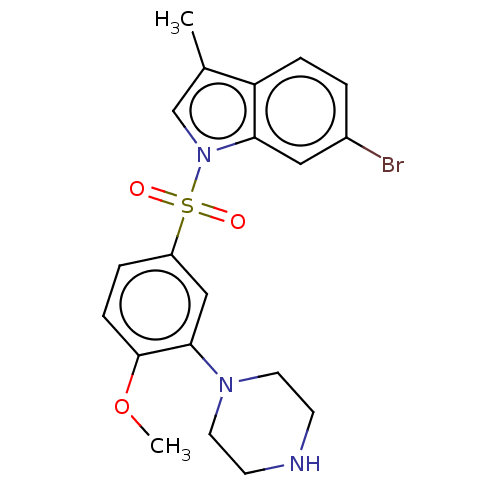
(CHEMBL5206617)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C)c2ccc(Br)cc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328389

(3-(difluoromethyl)-6-fluoro-1-((4-methoxy-3-(piper...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C(F)F)c2ccc(F)cc12 Show InChI InChI=1S/C20H20F3N3O3S/c1-29-19-5-3-14(11-18(19)25-8-6-24-7-9-25)30(27,28)26-12-16(20(22)23)15-4-2-13(21)10-17(15)26/h2-5,10-12,20,24H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50443101

(Cariprazine | RGH-188)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(-.77,5.69,;.57,4.92,;1.9,5.69,;.57,3.38,;-.77,2.61,;1.9,2.61,;1.9,1.07,;3.23,.3,;3.23,-1.24,;1.9,-2.01,;1.9,-3.55,;3.23,-4.32,;3.23,-5.86,;4.57,-6.63,;4.57,-8.17,;3.23,-8.94,;1.9,-8.17,;1.9,-6.63,;3.23,-10.48,;4.57,-11.25,;4.57,-12.79,;3.23,-13.56,;1.9,-12.79,;.57,-13.56,;1.9,-11.25,;.57,-10.48,;.57,-1.24,;.57,.3,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.269 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-SCH23390 from D3 receptor (unknown origin) expressed in HEK293T cell membranes measured after 2 hrs by microbeta scintillation c... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01327
BindingDB Entry DOI: 10.7270/Q2BG2SS4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50312840
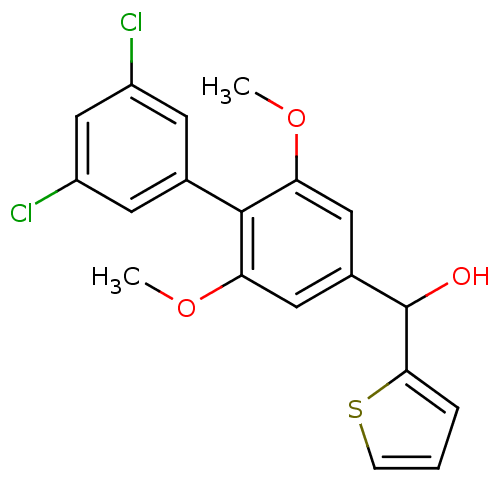
(CHEMBL1076680 | US9139546, 16 | [11C](3',5'-dichlo...)Show SMILES COc1cc(cc(OC)c1-c1cc(Cl)cc(Cl)c1)C(O)c1cccs1 Show InChI InChI=1S/C19H16Cl2O3S/c1-23-15-8-12(19(22)17-4-3-5-25-17)9-16(24-2)18(15)11-6-13(20)10-14(21)7-11/h3-10,19,22H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to rat brain CB2 receptor |
Bioorg Med Chem Lett 20: 1565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.074
BindingDB Entry DOI: 10.7270/Q2N29X34 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50443101

(Cariprazine | RGH-188)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(-.77,5.69,;.57,4.92,;1.9,5.69,;.57,3.38,;-.77,2.61,;1.9,2.61,;1.9,1.07,;3.23,.3,;3.23,-1.24,;1.9,-2.01,;1.9,-3.55,;3.23,-4.32,;3.23,-5.86,;4.57,-6.63,;4.57,-8.17,;3.23,-8.94,;1.9,-8.17,;1.9,-6.63,;3.23,-10.48,;4.57,-11.25,;4.57,-12.79,;3.23,-13.56,;1.9,-12.79,;.57,-13.56,;1.9,-11.25,;.57,-10.48,;.57,-1.24,;.57,.3,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-SCH23390 from D3 receptor (unknown origin) expressed in HEK293T cell membranes measured after 2 hrs by microbeta scintillation c... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01327
BindingDB Entry DOI: 10.7270/Q2BG2SS4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50395059
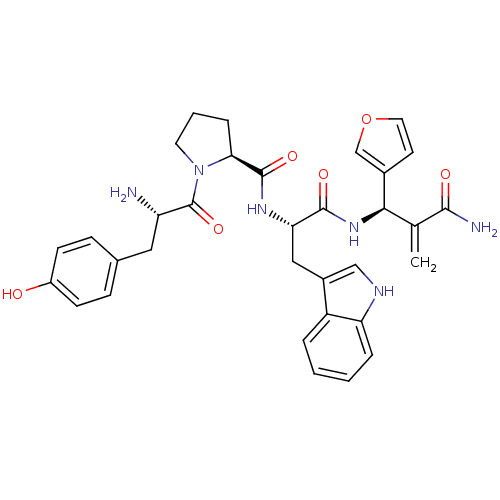
(CHEMBL2163917)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccoc1 |r| Show InChI InChI=1S/C33H36N6O6/c1-19(30(35)41)29(21-12-14-45-18-21)38-31(42)27(16-22-17-36-26-6-3-2-5-24(22)26)37-32(43)28-7-4-13-39(28)33(44)25(34)15-20-8-10-23(40)11-9-20/h2-3,5-6,8-12,14,17-18,25,27-29,36,40H,1,4,7,13,15-16,34H2,(H2,35,41)(H,37,43)(H,38,42)/t25-,27-,28-,29+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.274 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from FLAG-tagged mu opioid receptor expressed in HEK293 cells after 60 mins by liquid scintillation counter |
J Med Chem 55: 6224-36 (2012)
Article DOI: 10.1021/jm300664y
BindingDB Entry DOI: 10.7270/Q2T154S8 |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50235631
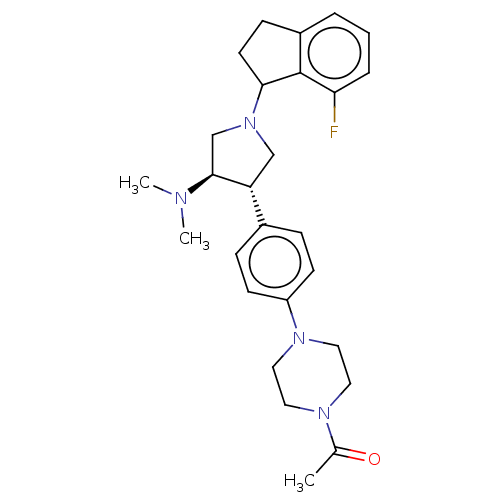
(CHEMBL4060827)Show SMILES CN(C)[C@H]1CN(C[C@@H]1c1ccc(cc1)N1CCN(CC1)C(C)=O)C1CCc2cccc(F)c12 |r| Show InChI InChI=1S/C27H35FN4O/c1-19(33)30-13-15-31(16-14-30)22-10-7-20(8-11-22)23-17-32(18-26(23)29(2)3)25-12-9-21-5-4-6-24(28)27(21)25/h4-8,10-11,23,25-26H,9,12-18H2,1-3H3/t23-,25?,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to EED (unknown origin) by TR-FRET based binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01344
BindingDB Entry DOI: 10.7270/Q23R0XMC |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50430807
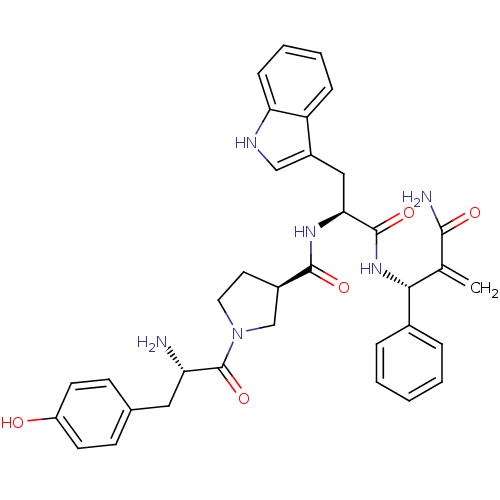
(CHEMBL2334770)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CC[C@H](C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccccc1 |r| Show InChI InChI=1S/C35H38N6O5/c1-21(32(37)43)31(23-7-3-2-4-8-23)40-34(45)30(18-25-19-38-29-10-6-5-9-27(25)29)39-33(44)24-15-16-41(20-24)35(46)28(36)17-22-11-13-26(42)14-12-22/h2-14,19,24,28,30-31,38,42H,1,15-18,20,36H2,(H2,37,43)(H,39,44)(H,40,45)/t24-,28+,30+,31-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.296 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... |
J Med Chem 56: 3102-14 (2013)
Article DOI: 10.1021/jm400195y
BindingDB Entry DOI: 10.7270/Q2KS6SW8 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18792
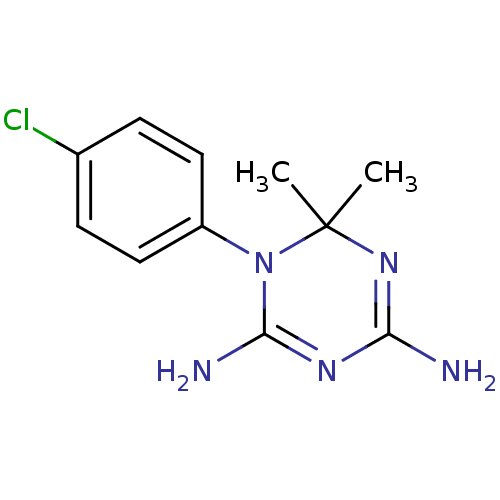
(1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...)Show InChI InChI=1S/C11H14ClN5/c1-11(2)16-9(13)15-10(14)17(11)8-5-3-7(12)4-6-8/h3-6H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | -54.4 | 37 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University
| Assay Description
The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... |
Nat Struct Biol 10: 257-65 (2003)
Article DOI: 10.1038/nsb921
BindingDB Entry DOI: 10.7270/Q2HH6HBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50252829

((2S,3S,4R,5R)-5-(6-(3-iodobenzylamino)-9H-purin-9-...)Show SMILES CNC(=O)[C@H]1S[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O3S/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human A3 adenosine receptor expressed in CHO cell membranes after 60 mins by gamma counting method |
J Med Chem 60: 7459-7475 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00805
BindingDB Entry DOI: 10.7270/Q2XK8HQH |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049547
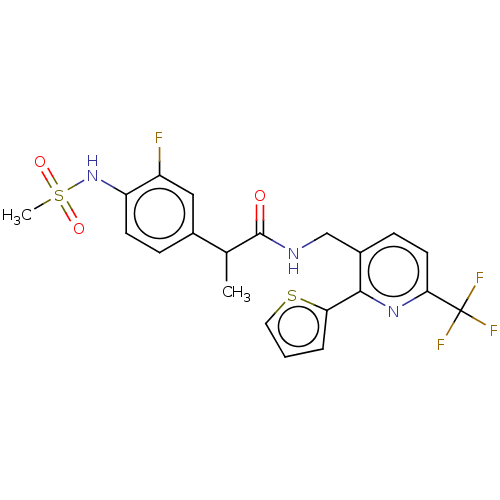
(CHEMBL3317484)Show SMILES CC(C(=O)NCc1ccc(nc1-c1cccs1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C21H19F4N3O3S2/c1-12(13-5-7-16(15(22)10-13)28-33(2,30)31)20(29)26-11-14-6-8-18(21(23,24)25)27-19(14)17-4-3-9-32-17/h3-10,12,28H,11H2,1-2H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced channel activation by FLIPR assay |
Bioorg Med Chem Lett 24: 4044-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.072
BindingDB Entry DOI: 10.7270/Q2JH3NTH |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315206
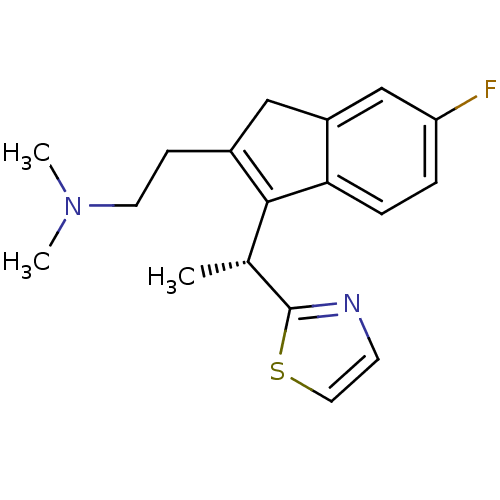
((R)-2-(6-fluoro-3-(1-(thiazol-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)C)Cc2cc(F)ccc12)c1nccs1 |r,c:2| Show InChI InChI=1S/C18H21FN2S/c1-12(18-20-7-9-22-18)17-13(6-8-21(2)3)10-14-11-15(19)4-5-16(14)17/h4-5,7,9,11-12H,6,8,10H2,1-3H3/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
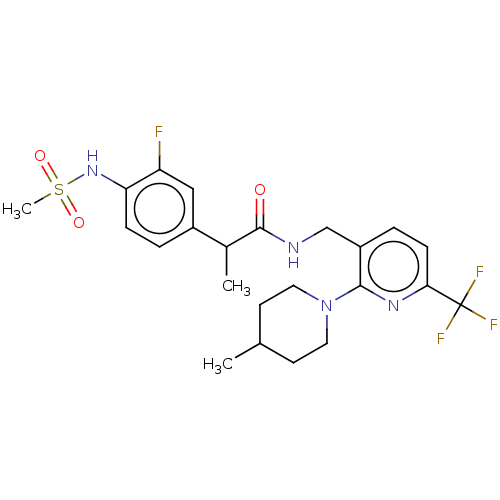
(Homo sapiens (Human)) | BDBM50049553
(CHEMBL2177428)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced channel activation by FLIPR assay |
Bioorg Med Chem Lett 24: 4044-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.072
BindingDB Entry DOI: 10.7270/Q2JH3NTH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data