| Reaction Details |
|---|
 | Report a problem with these data |
| Target | C-C chemokine receptor type 1 |
|---|
| Ligand | BDBM50144430 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_86826 (CHEMBL694697) |
|---|
| IC50 | 440±n/a nM |
|---|
| Citation |  Brown, MF; Avery, M; Brissette, WH; Chang, JH; Colizza, K; Conklyn, M; DiRico, AP; Gladue, RP; Kath, JC; Krueger, SS; Lira, PD; Lillie, BM; Lundquist, GD; Mairs, EN; McElroy, EB; McGlynn, MA; Paradis, TJ; Poss, CS; Rossulek, MI; Shepard, RM; Sims, J; Strelevitz, TJ; Truesdell, S; Tylaska, LA; Yoon, K; Zheng, D Novel CCR1 antagonists with improved metabolic stability. Bioorg Med Chem Lett14:2175-9 (2004) [PubMed] Article Brown, MF; Avery, M; Brissette, WH; Chang, JH; Colizza, K; Conklyn, M; DiRico, AP; Gladue, RP; Kath, JC; Krueger, SS; Lira, PD; Lillie, BM; Lundquist, GD; Mairs, EN; McElroy, EB; McGlynn, MA; Paradis, TJ; Poss, CS; Rossulek, MI; Shepard, RM; Sims, J; Strelevitz, TJ; Truesdell, S; Tylaska, LA; Yoon, K; Zheng, D Novel CCR1 antagonists with improved metabolic stability. Bioorg Med Chem Lett14:2175-9 (2004) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| C-C chemokine receptor type 1 |
|---|
| Name: | C-C chemokine receptor type 1 |
|---|
| Synonyms: | C-C CKR-1 | C-C chemokine receptor type 1 (CCR1) | CC-CKR-1 | CCR-1 | CCR1 | CCR1_HUMAN | CD_antigen=CD191 | CMKBR1 | CMKR1 | HM145 | LD78 receptor | MIP-1alpha-R | Macrophage inflammatory protein 1-alpha receptor | RANTES-R | SCYAR1 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 41180.69 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P32246 |
|---|
| Residue: | 355 |
|---|
| Sequence: | METPNTTEDYDTTTEFDYGDATPCQKVNERAFGAQLLPPLYSLVFVIGLVGNILVVLVLV
QYKRLKNMTSIYLLNLAISDLLFLFTLPFWIDYKLKDDWVFGDAMCKILSGFYYTGLYSE
IFFIILLTIDRYLAIVHAVFALRARTVTFGVITSIIIWALAILASMPGLYFSKTQWEFTH
HTCSLHFPHESLREWKLFQALKLNLFGLVLPLLVMIICYTGIIKILLRRPNEKKSKAVRL
IFVIMIIFFLFWTPYNLTILISVFQDFLFTHECEQSRHLDLAVQVTEVIAYTHCCVNPVI
YAFVGERFRKYLRQLFHRRVAVHLVKWLPFLSVDRLERVSSTSPSTGEHELSAGF
|
|
|
|---|
| BDBM50144430 |
|---|
| n/a |
|---|
| Name | BDBM50144430 |
|---|
| Synonyms: | CHEMBL65784 | Quinoxaline-2-carboxylic acid ((1S,2S)-1-benzyl-4-benzylcarbamoyl-7-fluoro-2-hydroxy-7-methyl-octyl)-amide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C33H37FN4O3 |
|---|
| Mol. Mass. | 556.6703 |
|---|
| SMILES | CC(C)(F)CCC(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C(=O)NCc1ccccc1 |
|---|
| Structure | 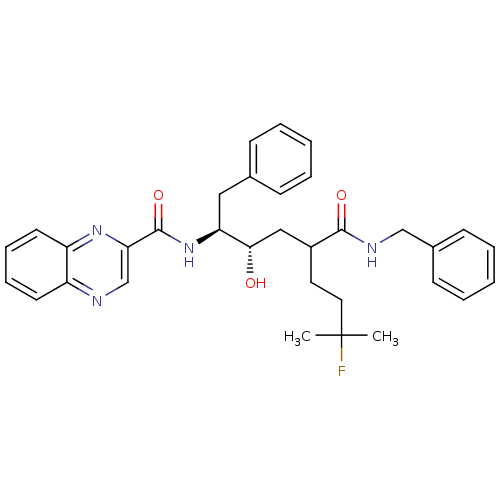
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Brown, MF; Avery, M; Brissette, WH; Chang, JH; Colizza, K; Conklyn, M; DiRico, AP; Gladue, RP; Kath, JC; Krueger, SS; Lira, PD; Lillie, BM; Lundquist, GD; Mairs, EN; McElroy, EB; McGlynn, MA; Paradis, TJ; Poss, CS; Rossulek, MI; Shepard, RM; Sims, J; Strelevitz, TJ; Truesdell, S; Tylaska, LA; Yoon, K; Zheng, D Novel CCR1 antagonists with improved metabolic stability. Bioorg Med Chem Lett14:2175-9 (2004) [PubMed] Article
Brown, MF; Avery, M; Brissette, WH; Chang, JH; Colizza, K; Conklyn, M; DiRico, AP; Gladue, RP; Kath, JC; Krueger, SS; Lira, PD; Lillie, BM; Lundquist, GD; Mairs, EN; McElroy, EB; McGlynn, MA; Paradis, TJ; Poss, CS; Rossulek, MI; Shepard, RM; Sims, J; Strelevitz, TJ; Truesdell, S; Tylaska, LA; Yoon, K; Zheng, D Novel CCR1 antagonists with improved metabolic stability. Bioorg Med Chem Lett14:2175-9 (2004) [PubMed] Article