| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tyrosine-protein kinase BTK |
|---|
| Ligand | BDBM50589212 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2192545 (CHEMBL5104905) |
|---|
| IC50 | 0.800000±n/a nM |
|---|
| Citation |  Owens, TD; Brameld, KA; Verner, EJ; Ton, T; Li, X; Zhu, J; Masjedizadeh, MR; Bradshaw, JM; Hill, RJ; Tam, D; Bisconte, A; Kim, EO; Francesco, M; Xing, Y; Shu, J; Karr, D; LaStant, J; Finkle, D; Loewenstein, N; Haberstock-Debic, H; Taylor, MJ; Nunn, P; Langrish, CL; Goldstein, DM Discovery of Reversible Covalent Bruton's Tyrosine Kinase Inhibitors PRN473 and PRN1008 (Rilzabrutinib). J Med Chem65:5300-5316 (2022) [PubMed] Article Owens, TD; Brameld, KA; Verner, EJ; Ton, T; Li, X; Zhu, J; Masjedizadeh, MR; Bradshaw, JM; Hill, RJ; Tam, D; Bisconte, A; Kim, EO; Francesco, M; Xing, Y; Shu, J; Karr, D; LaStant, J; Finkle, D; Loewenstein, N; Haberstock-Debic, H; Taylor, MJ; Nunn, P; Langrish, CL; Goldstein, DM Discovery of Reversible Covalent Bruton's Tyrosine Kinase Inhibitors PRN473 and PRN1008 (Rilzabrutinib). J Med Chem65:5300-5316 (2022) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Tyrosine-protein kinase BTK |
|---|
| Name: | Tyrosine-protein kinase BTK |
|---|
| Synonyms: | AGMX1 | ATK | Agammaglobulinaemia tyrosine kinase | Agammaglobulinemia tyrosine kinase | B cell progenitor kinase | B-cell progenitor kinase | BPK | BTK | BTK_HUMAN | Bruton tyrosine kinase | Tyrosine Kinase BTK | Tyrosine-protein kinase (BTK) | Tyrosine-protein kinase BTK (BTK) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 76289.95 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q06187 |
|---|
| Residue: | 659 |
|---|
| Sequence: | MAAVILESIFLKRSQQKKKTSPLNFKKRLFLLTVHKLSYYEYDFERGRRGSKKGSIDVEK
ITCVETVVPEKNPPPERQIPRRGEESSEMEQISIIERFPYPFQVVYDEGPLYVFSPTEEL
RKRWIHQLKNVIRYNSDLVQKYHPCFWIDGQYLCCSQTAKNAMGCQILENRNGSLKPGSS
HRKTKKPLPPTPEEDQILKKPLPPEPAAAPVSTSELKKVVALYDYMPMNANDLQLRKGDE
YFILEESNLPWWRARDKNGQEGYIPSNYVTEAEDSIEMYEWYSKHMTRSQAEQLLKQEGK
EGGFIVRDSSKAGKYTVSVFAKSTGDPQGVIRHYVVCSTPQSQYYLAEKHLFSTIPELIN
YHQHNSAGLISRLKYPVSQQNKNAPSTAGLGYGSWEIDPKDLTFLKELGTGQFGVVKYGK
WRGQYDVAIKMIKEGSMSEDEFIEEAKVMMNLSHEKLVQLYGVCTKQRPIFIITEYMANG
CLLNYLREMRHRFQTQQLLEMCKDVCEAMEYLESKQFLHRDLAARNCLVNDQGVVKVSDF
GLSRYVLDDEYTSSVGSKFPVRWSPPEVLMYSKFSSKSDIWAFGVLMWEIYSLGKMPYER
FTNSETAEHIAQGLRLYRPHLASEKVYTIMYSCWHEKADERPTFKILLSNILDVMDEES
|
|
|
|---|
| BDBM50589212 |
|---|
| n/a |
|---|
| Name | BDBM50589212 |
|---|
| Synonyms: | CHEMBL5195816 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C36H42FN9O2 |
|---|
| Mol. Mass. | 651.7762 |
|---|
| SMILES | C[C@H]1CN(C[C@@H](C)N1C)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| |
|---|
| Structure | 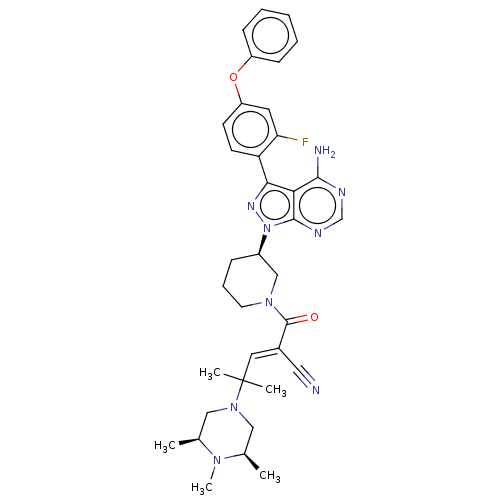
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Owens, TD; Brameld, KA; Verner, EJ; Ton, T; Li, X; Zhu, J; Masjedizadeh, MR; Bradshaw, JM; Hill, RJ; Tam, D; Bisconte, A; Kim, EO; Francesco, M; Xing, Y; Shu, J; Karr, D; LaStant, J; Finkle, D; Loewenstein, N; Haberstock-Debic, H; Taylor, MJ; Nunn, P; Langrish, CL; Goldstein, DM Discovery of Reversible Covalent Bruton's Tyrosine Kinase Inhibitors PRN473 and PRN1008 (Rilzabrutinib). J Med Chem65:5300-5316 (2022) [PubMed] Article
Owens, TD; Brameld, KA; Verner, EJ; Ton, T; Li, X; Zhu, J; Masjedizadeh, MR; Bradshaw, JM; Hill, RJ; Tam, D; Bisconte, A; Kim, EO; Francesco, M; Xing, Y; Shu, J; Karr, D; LaStant, J; Finkle, D; Loewenstein, N; Haberstock-Debic, H; Taylor, MJ; Nunn, P; Langrish, CL; Goldstein, DM Discovery of Reversible Covalent Bruton's Tyrosine Kinase Inhibitors PRN473 and PRN1008 (Rilzabrutinib). J Med Chem65:5300-5316 (2022) [PubMed] Article