

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Escherichia coli) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026300 (6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026300 (6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026308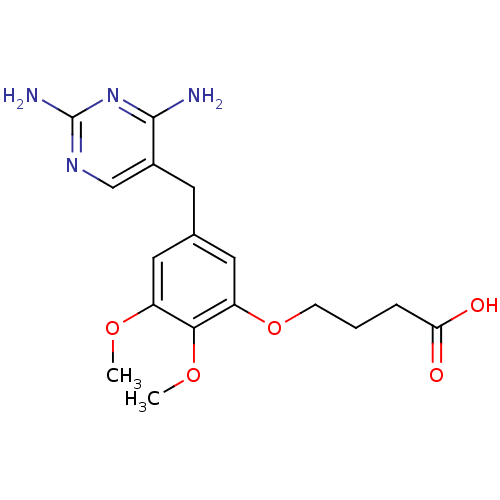 (4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026308 (4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026318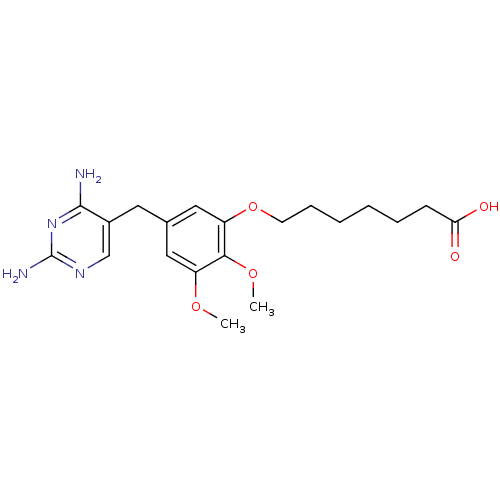 (7-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026318 (7-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026314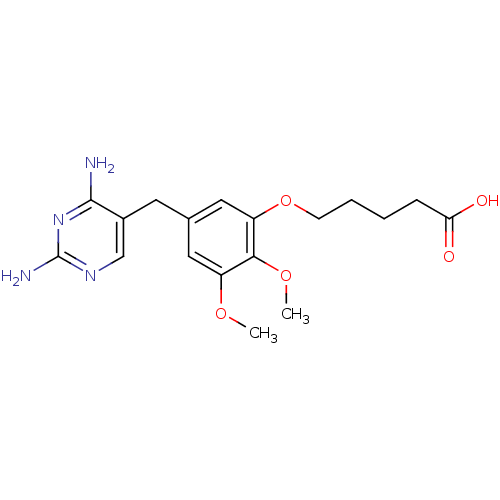 (5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026314 (5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50345553 (3-(2-(2-methylthiazol-4-yl)ethynyl)-5-bromobenzoni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Neurodegenerative Disorders Curated by ChEMBL | Assay Description Displacement of [3H]methoxy-PEPY from rat mGluR5 expressed in human HEK-293 cells by liquid scintillation counting | Bioorg Med Chem Lett 21: 3243-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.047 BindingDB Entry DOI: 10.7270/Q290244M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50345552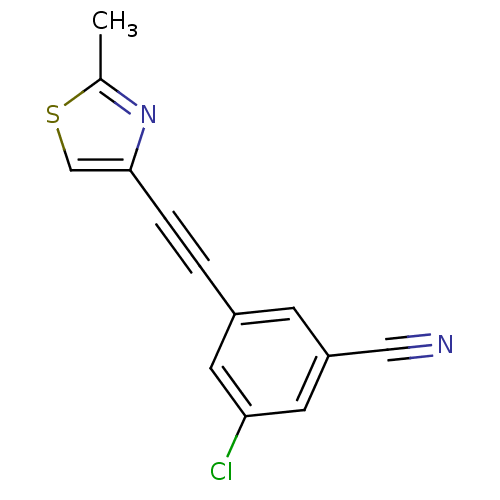 (3-(2-(2-methylthiazol-4-yl)ethynyl)-5-chlorobenzon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Neurodegenerative Disorders Curated by ChEMBL | Assay Description Displacement of [3H]methoxy-PEPY from rat mGluR5 expressed in human HEK-293 cells by liquid scintillation counting | Bioorg Med Chem Lett 21: 3243-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.047 BindingDB Entry DOI: 10.7270/Q290244M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50345572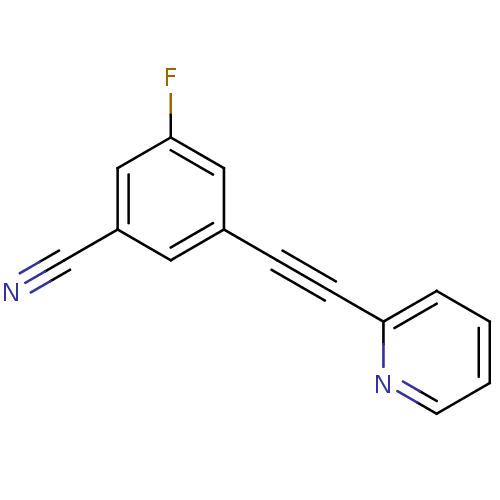 (3-(2-(pyridin-2-yl)ethynyl)-5-fluorobenzonitrile |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Neurodegenerative Disorders Curated by ChEMBL | Assay Description Displacement of [3H]methoxy-PEPY from rat mGluR5 expressed in human HEK-293 cells by liquid scintillation counting | Bioorg Med Chem Lett 21: 3243-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.047 BindingDB Entry DOI: 10.7270/Q290244M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50160248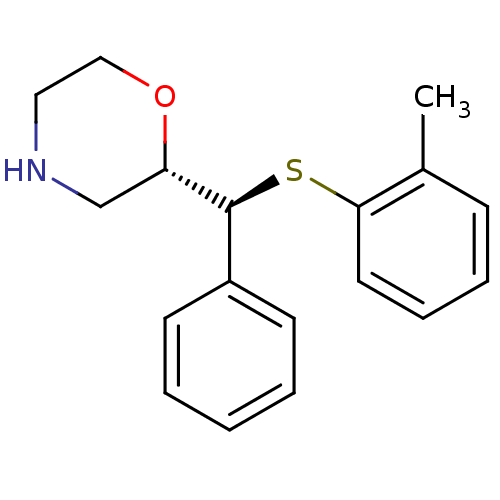 ((2S,3S)-2-[alpha-(2-Methylphenylthio)phenylmethyl]...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human NET receptor expressed in HEK293 cells | J Med Chem 52: 62-73 (2009) Article DOI: 10.1021/jm800817h BindingDB Entry DOI: 10.7270/Q2CN74TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50345555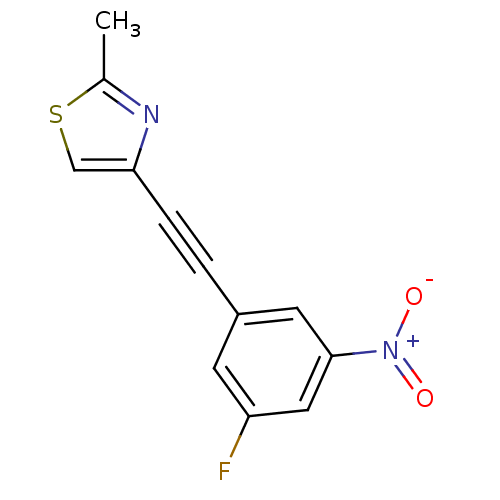 (3-(2-(2-methylthiazol-4-yl)ethynyl)-5-nitrofluorob...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Neurodegenerative Disorders Curated by ChEMBL | Assay Description Displacement of [3H]methoxy-PEPY from rat mGluR5 expressed in human HEK-293 cells by liquid scintillation counting | Bioorg Med Chem Lett 21: 3243-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.047 BindingDB Entry DOI: 10.7270/Q290244M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50258612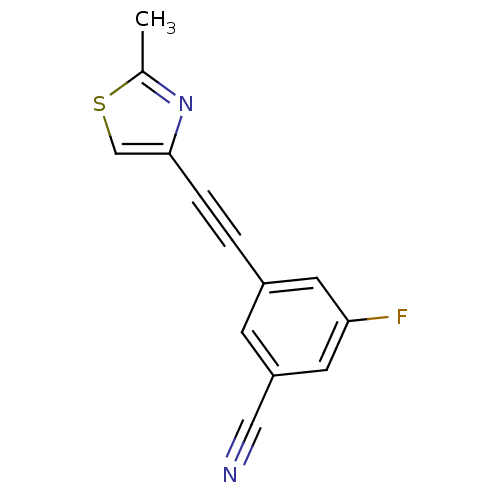 (3-Fluoro-5-cyano-1-(2-methylthiazol-4-ylethynyl)be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Neurodegenerative Disorders Curated by ChEMBL | Assay Description Displacement of [3H]methoxy-PEPY from rat mGluR5 expressed in human HEK-293 cells by liquid scintillation counting | Bioorg Med Chem Lett 21: 3243-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.047 BindingDB Entry DOI: 10.7270/Q290244M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50345550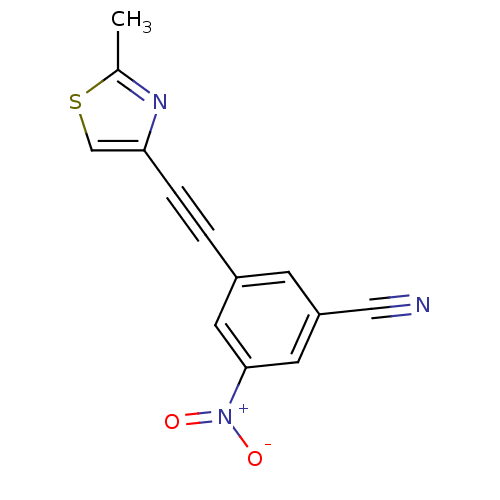 (3-(2-(2-Methylthiazol-4-yl)ethynyl)-5-nitrobenzoni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Neurodegenerative Disorders Curated by ChEMBL | Assay Description Displacement of [3H]methoxy-PEPY from rat mGluR5 expressed in human HEK-293 cells by liquid scintillation counting | Bioorg Med Chem Lett 21: 3243-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.047 BindingDB Entry DOI: 10.7270/Q290244M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026307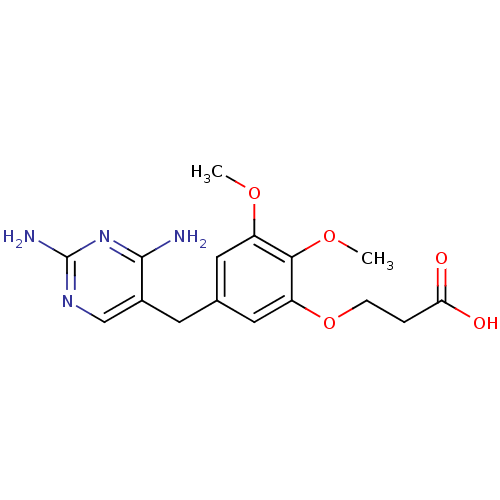 (3-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026307 (3-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM22166 (3-(4-iodophenyl)tropane-2-carboxylic acid methyl e...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Displacement of [3H]cyanoimipramine from SERT in rat cerebral cortex homogenate | Bioorg Med Chem Lett 16: 5222-5 (2006) Article DOI: 10.1016/j.bmcl.2006.07.013 BindingDB Entry DOI: 10.7270/Q2N29WMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026317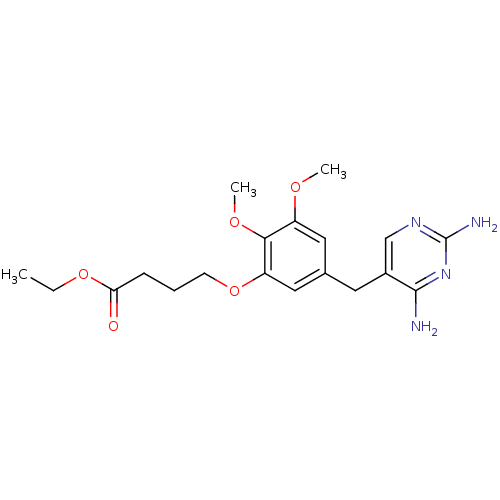 (4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026317 (4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50077906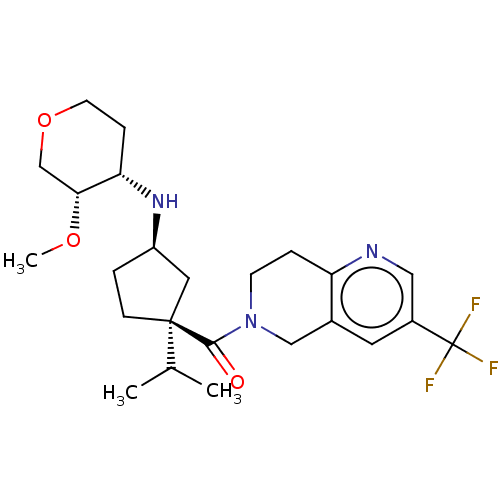 (CHEMBL3305901) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of 0.1 nM [125I]CCL2 from human CCR2 expressing human U2OS cell membrane by scintillation spectrometry | Eur J Med Chem 93: 121-34 (2015) Article DOI: 10.1016/j.ejmech.2015.01.063 BindingDB Entry DOI: 10.7270/Q25M67DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50345558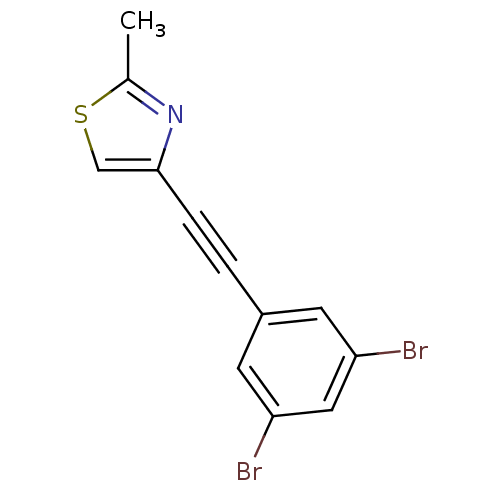 (3-(2-(2-methylthiazol-4-yl)ethynyl)-1,5-dibromoben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Neurodegenerative Disorders Curated by ChEMBL | Assay Description Displacement of [3H]methoxy-PEPY from rat mGluR5 expressed in human HEK-293 cells by liquid scintillation counting | Bioorg Med Chem Lett 21: 3243-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.047 BindingDB Entry DOI: 10.7270/Q290244M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026316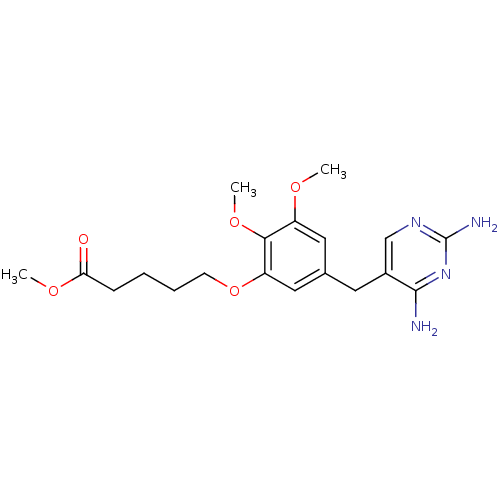 (5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026316 (5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50198230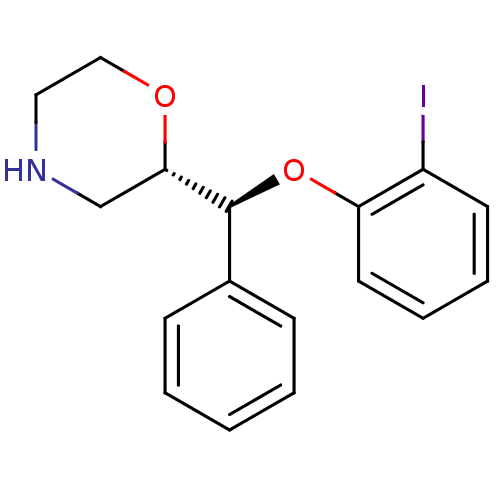 ((S)-2-((S)-(2-iodophenoxy)(phenyl)methyl)morpholin...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Degenerative Diseases Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from NET in rat brain membranes | Bioorg Med Chem Lett 17: 533-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.018 BindingDB Entry DOI: 10.7270/Q2WS8SX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026306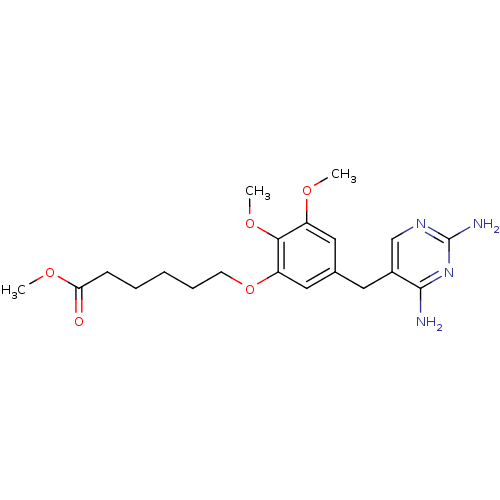 (6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase Inhibitor of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026306 (6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50345551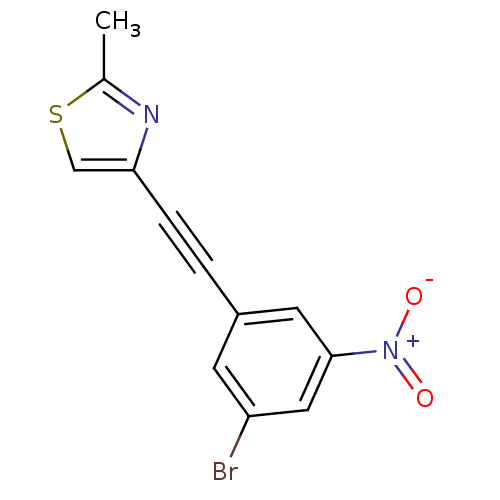 (3-(2-(2-methylthiazol-4-yl)ethynyl)-5-nitrobromobe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Neurodegenerative Disorders Curated by ChEMBL | Assay Description Displacement of [3H]methoxy-PEPY from rat mGluR5 expressed in human HEK-293 cells by liquid scintillation counting | Bioorg Med Chem Lett 21: 3243-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.047 BindingDB Entry DOI: 10.7270/Q290244M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50199005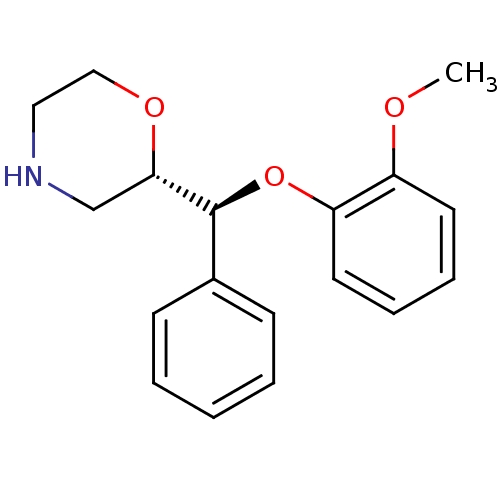 ((S)-2-((S)-(2-methoxyphenoxy)(phenyl)methyl)morpho...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human NET receptor expressed in HEK293 cells | J Med Chem 52: 62-73 (2009) Article DOI: 10.1021/jm800817h BindingDB Entry DOI: 10.7270/Q2CN74TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM22166 (3-(4-iodophenyl)tropane-2-carboxylic acid methyl e...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Displacement of [3H]beta-CIT from DAT in rat caudate homogenate | Bioorg Med Chem Lett 16: 5222-5 (2006) Article DOI: 10.1016/j.bmcl.2006.07.013 BindingDB Entry DOI: 10.7270/Q2N29WMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50254209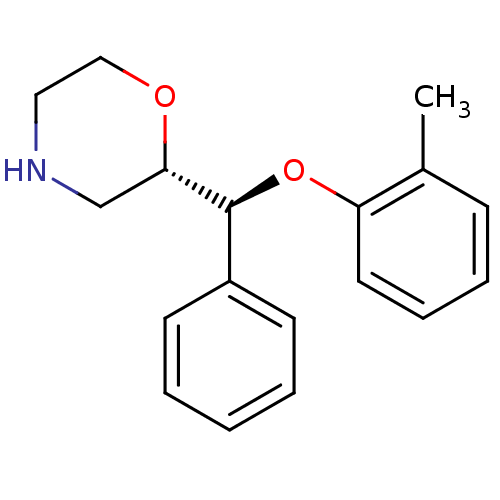 ((2S,3S)-2-[alpha-(2-Methylphenoxy)phenylmethyl]mor...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human NET receptor expressed in HEK293 cells | J Med Chem 52: 62-73 (2009) Article DOI: 10.1021/jm800817h BindingDB Entry DOI: 10.7270/Q2CN74TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50370475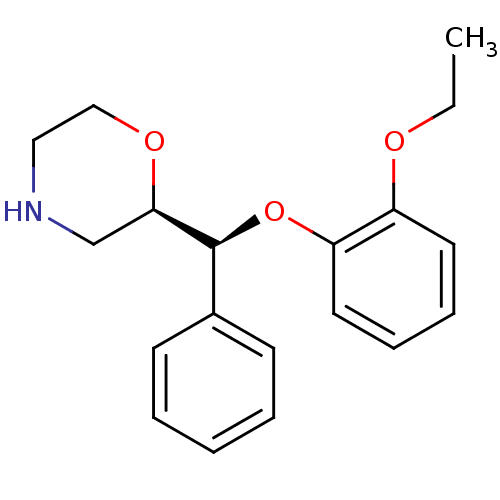 (S,S-REBOXETINE) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human NET receptor expressed in HEK293 cells | J Med Chem 52: 62-73 (2009) Article DOI: 10.1021/jm800817h BindingDB Entry DOI: 10.7270/Q2CN74TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50345557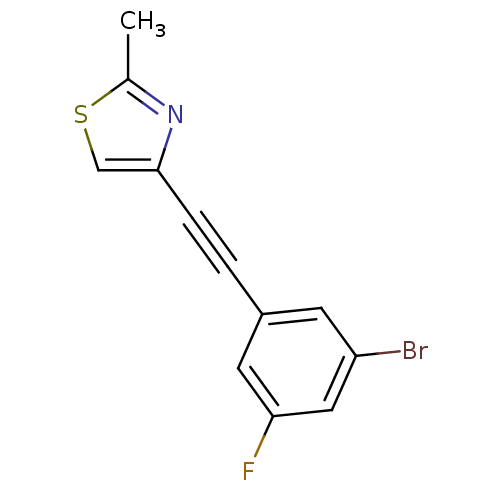 (3-(2-(2-methylthiazol-4-yl)ethynyl)-5-bromofluorob...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Neurodegenerative Disorders Curated by ChEMBL | Assay Description Displacement of [3H]methoxy-PEPY from rat mGluR5 expressed in human HEK-293 cells by liquid scintillation counting | Bioorg Med Chem Lett 21: 3243-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.047 BindingDB Entry DOI: 10.7270/Q290244M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50077924 (CHEMBL3417124) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of 0.1 nM [125I]CCL2 from human CCR2 expressing human U2OS cell membrane by scintillation spectrometry | Eur J Med Chem 93: 121-34 (2015) Article DOI: 10.1016/j.ejmech.2015.01.063 BindingDB Entry DOI: 10.7270/Q25M67DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM286984 (8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human FGFR2 preincubated with enzyme followed by peptide substrate addition by caliper capillary electrophoresis method | J Med Chem 60: 6516-6527 (2017) Article DOI: 10.1021/acs.jmedchem.7b00360 BindingDB Entry DOI: 10.7270/Q2C53P3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50077980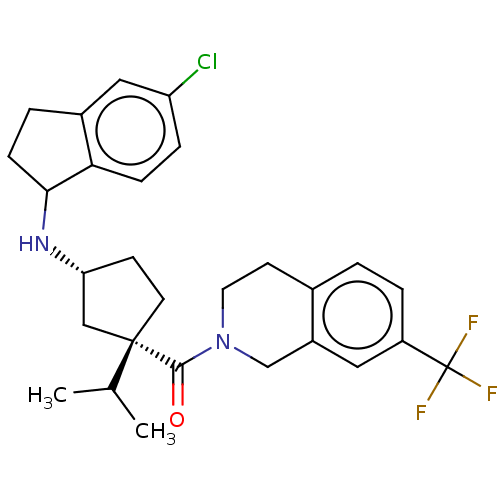 (CHEMBL3417117) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of 0.1 nM [125I]CCL2 from human CCR2 expressing human U2OS cell membrane by scintillation spectrometry | Eur J Med Chem 93: 121-34 (2015) Article DOI: 10.1016/j.ejmech.2015.01.063 BindingDB Entry DOI: 10.7270/Q25M67DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM286984 (8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human FGFR1 using 5-FAM-KKKKEEIYFFF-NH2 as substrate preincubated with enzyme followed by peptide substrate addition by ca... | J Med Chem 60: 6516-6527 (2017) Article DOI: 10.1021/acs.jmedchem.7b00360 BindingDB Entry DOI: 10.7270/Q2C53P3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50160633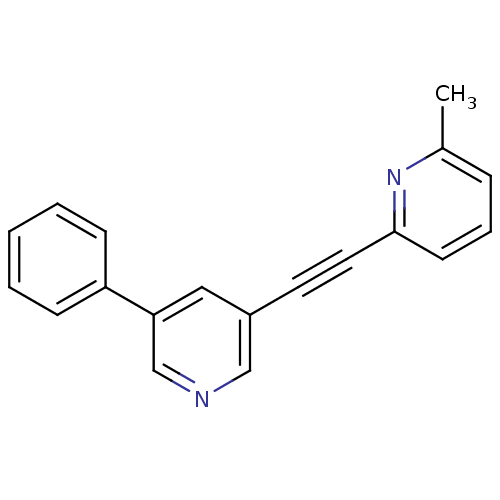 (2-methyl-6-[(5-phenylpyridin-3-yl)ethynyl]pyridine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description In vitro binding affinity of compound towards rat metabotropic glutamate receptor 5 was determined using inositol phosphate hydrolysis assay | Bioorg Med Chem Lett 15: 945-9 (2005) Article DOI: 10.1016/j.bmcl.2004.12.047 BindingDB Entry DOI: 10.7270/Q2QN6681 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50345556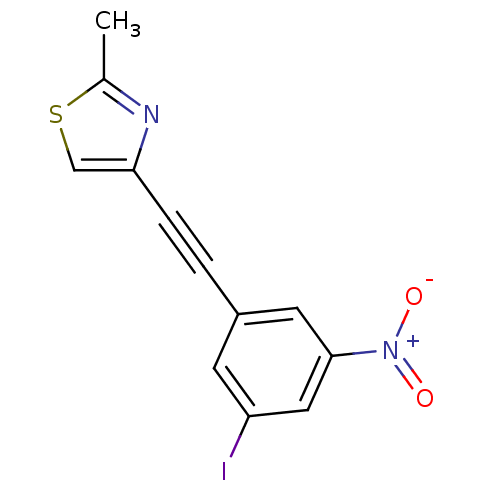 (3-(2-(2-methylthiazol-4-yl)ethynyl)-5-nitroiodoben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Neurodegenerative Disorders Curated by ChEMBL | Assay Description Displacement of [3H]methoxy-PEPY from rat mGluR5 expressed in human HEK-293 cells by liquid scintillation counting | Bioorg Med Chem Lett 21: 3243-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.047 BindingDB Entry DOI: 10.7270/Q290244M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026304 (7-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026304 (7-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50077912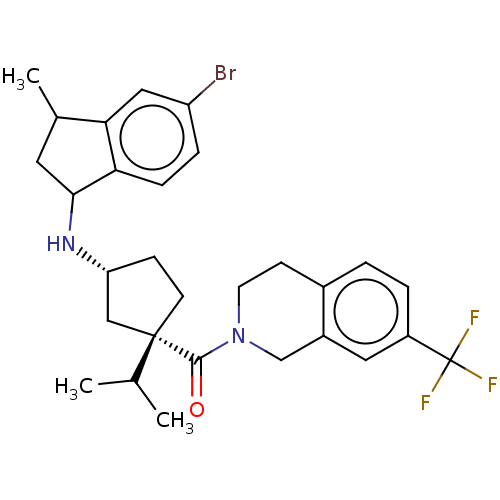 (CHEMBL3417231) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of 0.1 nM [125I]CCL2 from human CCR2 expressing human U2OS cell membrane by scintillation spectrometry | Eur J Med Chem 93: 121-34 (2015) Article DOI: 10.1016/j.ejmech.2015.01.063 BindingDB Entry DOI: 10.7270/Q25M67DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50077991 (CHEMBL3417237) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of 0.1 nM [125I]CCL2 from human CCR2 expressing human U2OS cell membrane by scintillation spectrometry | Eur J Med Chem 93: 121-34 (2015) Article DOI: 10.1016/j.ejmech.2015.01.063 BindingDB Entry DOI: 10.7270/Q25M67DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50345554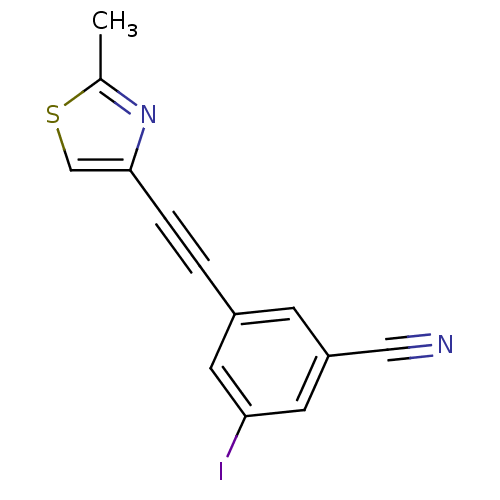 (3-(2-(2-methylthiazol-4-yl)ethynyl)-5-iodobenzonit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Neurodegenerative Disorders Curated by ChEMBL | Assay Description Displacement of [3H]methoxy-PEPY from rat mGluR5 expressed in human HEK-293 cells by liquid scintillation counting | Bioorg Med Chem Lett 21: 3243-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.047 BindingDB Entry DOI: 10.7270/Q290244M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50077986 (CHEMBL3417111) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of 0.1 nM [125I]CCL2 from human CCR2 expressing human U2OS cell membrane by scintillation spectrometry | Eur J Med Chem 93: 121-34 (2015) Article DOI: 10.1016/j.ejmech.2015.01.063 BindingDB Entry DOI: 10.7270/Q25M67DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM286984 (8-(3-(4-acryloylpiperazin-1-yl)propyl)-6-(2,6-dich...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Principia Biopharma, Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human FGFR3 preincubated with enzyme followed by peptide substrate addition by caliper capillary electrophoresis method | J Med Chem 60: 6516-6527 (2017) Article DOI: 10.1021/acs.jmedchem.7b00360 BindingDB Entry DOI: 10.7270/Q2C53P3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026313 (5-[4-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,6-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1846 total ) | Next | Last >> |