 Found 140 hits with Last Name = 'taylor' and Initial = 'mj'
Found 140 hits with Last Name = 'taylor' and Initial = 'mj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Squalene synthase
(Rattus norvegicus) | BDBM50291312
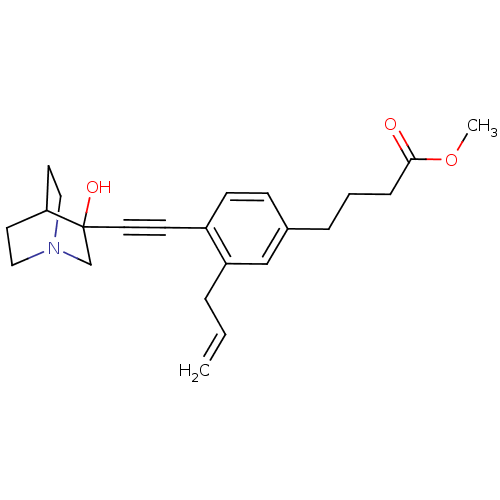
(4-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES COC(=O)CCCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:12:13:18.17:20.21,14:13:18.17:20.21,(20.76,-2.86,;19.42,-2.1,;18.08,-2.89,;18.1,-4.43,;16.75,-2.12,;15.43,-2.9,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.23,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.63,;3.26,-8.24,;3.14,-6.74,;4.77,-6.14,;4.96,-4.38,;5.36,-5.6,;10.08,-2.93,;8.76,-2.17,;7.43,-2.94,;6.09,-2.18,;11.42,-2.15,)| Show InChI InChI=1S/C23H29NO3/c1-3-5-20-16-18(6-4-7-22(25)27-2)8-9-19(20)10-13-23(26)17-24-14-11-21(23)12-15-24/h3,8-9,16,21,26H,1,4-7,11-12,14-15,17H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50075719
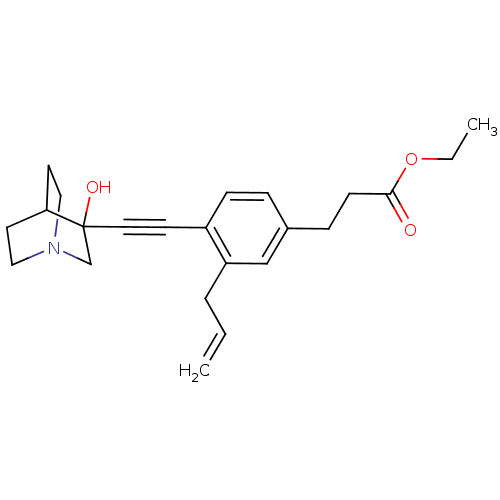
(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES CCOC(=O)CCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:12:13:18.17:20.21,14:13:18.17:20.21,(10.63,-1.41,;9.54,-2.5,;9.94,-3.98,;8.86,-5.08,;9.25,-6.57,;7.36,-4.68,;6.27,-5.77,;4.78,-5.38,;4.38,-3.89,;2.9,-3.49,;1.81,-4.59,;.33,-4.19,;-1.17,-3.79,;-2.66,-3.4,;-2.26,-1.9,;-3.89,-4.07,;-4.88,-2.49,;-6.75,-2.53,;-5.51,-1.8,;-3.62,-1.88,;-3.15,-.9,;-4.25,-1.27,;2.22,-6.06,;1.13,-7.15,;-.37,-6.75,;-1.45,-7.84,;3.7,-6.46,)| Show InChI InChI=1S/C23H29NO3/c1-3-5-20-16-18(7-9-22(25)27-4-2)6-8-19(20)10-13-23(26)17-24-14-11-21(23)12-15-24/h3,6,8,16,21,26H,1,4-5,7,9,11-12,14-15,17H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291315

(5-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES COC(=O)CCCCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:13:14:19.18:21.22,15:14:19.18:21.22,(22.09,-2.1,;20.76,-2.86,;19.42,-2.1,;19.42,-.56,;18.08,-2.89,;16.75,-2.12,;15.43,-2.9,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.23,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.63,;3.26,-8.24,;3.14,-6.74,;4.77,-6.14,;4.96,-4.38,;5.36,-5.6,;10.08,-2.93,;8.76,-2.17,;7.43,-2.94,;6.09,-2.18,;11.42,-2.15,)| Show InChI InChI=1S/C24H31NO3/c1-3-6-21-17-19(7-4-5-8-23(26)28-2)9-10-20(21)11-14-24(27)18-25-15-12-22(24)13-16-25/h3,9-10,17,22,27H,1,4-8,12-13,15-16,18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291311

(6-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES COC(=O)CCCCCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:14:15:20.19:22.23,16:15:20.19:22.23,(23.43,-2.86,;22.09,-2.1,;20.76,-2.86,;20.76,-4.4,;19.42,-2.1,;18.08,-2.89,;16.75,-2.12,;15.43,-2.9,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.23,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.63,;3.26,-8.24,;3.14,-6.74,;4.77,-6.14,;4.96,-4.38,;5.36,-5.6,;10.08,-2.93,;8.76,-2.17,;7.43,-2.94,;6.09,-2.18,;11.42,-2.15,)| Show InChI InChI=1S/C25H33NO3/c1-3-7-22-18-20(8-5-4-6-9-24(27)29-2)10-11-21(22)12-15-25(28)19-26-16-13-23(25)14-17-26/h3,10-11,18,23,28H,1,4-9,13-14,16-17,19H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291316
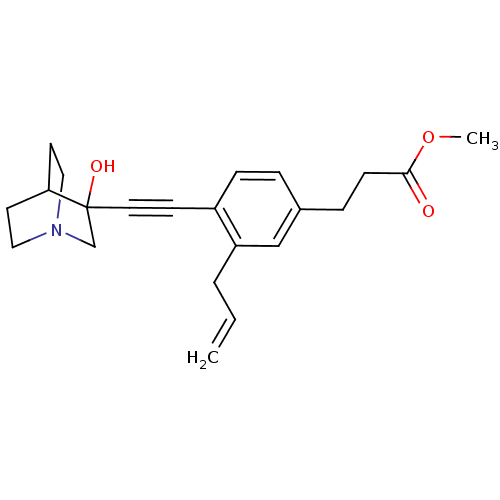
(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES COC(=O)CCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:11:12:17.16:19.20,13:12:17.16:19.20,(19.42,-2.1,;18.08,-2.89,;16.75,-2.12,;16.75,-.58,;15.43,-2.9,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.23,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.63,;3.26,-8.24,;3.14,-6.74,;4.77,-6.14,;4.96,-4.38,;5.36,-5.6,;10.08,-2.93,;8.76,-2.17,;7.43,-2.94,;6.09,-2.18,;11.42,-2.15,)| Show InChI InChI=1S/C22H27NO3/c1-3-4-19-15-17(6-8-21(24)26-2)5-7-18(19)9-12-22(25)16-23-13-10-20(22)11-14-23/h3,5,7,15,20,25H,1,4,6,8,10-11,13-14,16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Homo sapiens (Human)) | BDBM50075719

(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES CCOC(=O)CCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:12:13:18.17:20.21,14:13:18.17:20.21,(10.63,-1.41,;9.54,-2.5,;9.94,-3.98,;8.86,-5.08,;9.25,-6.57,;7.36,-4.68,;6.27,-5.77,;4.78,-5.38,;4.38,-3.89,;2.9,-3.49,;1.81,-4.59,;.33,-4.19,;-1.17,-3.79,;-2.66,-3.4,;-2.26,-1.9,;-3.89,-4.07,;-4.88,-2.49,;-6.75,-2.53,;-5.51,-1.8,;-3.62,-1.88,;-3.15,-.9,;-4.25,-1.27,;2.22,-6.06,;1.13,-7.15,;-.37,-6.75,;-1.45,-7.84,;3.7,-6.46,)| Show InChI InChI=1S/C23H29NO3/c1-3-5-20-16-18(7-9-22(25)27-4-2)6-8-19(20)10-13-23(26)17-24-14-11-21(23)12-15-24/h3,6,8,16,21,26H,1,4-5,7,9,11-12,14-15,17H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against human microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291317
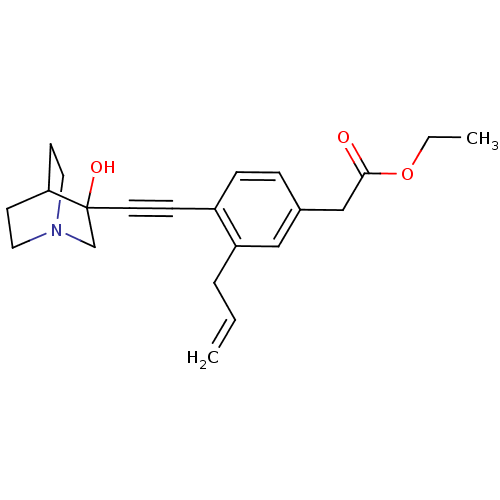
(CHEMBL154472 | [3-Allyl-4-(3-hydroxy-1-aza-bicyclo...)Show SMILES CCOC(=O)Cc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:11:12:17.16:19.20,13:12:17.16:19.20,(19.42,-2.1,;18.08,-2.89,;16.75,-2.12,;15.43,-2.9,;15.43,-4.44,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.23,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.63,;3.26,-8.24,;3.14,-6.74,;4.77,-6.14,;4.96,-4.38,;5.36,-5.6,;10.08,-2.93,;8.76,-2.17,;7.43,-2.94,;6.09,-2.18,;11.42,-2.15,)| Show InChI InChI=1S/C22H27NO3/c1-3-5-19-14-17(15-21(24)26-4-2)6-7-18(19)8-11-22(25)16-23-12-9-20(22)10-13-23/h3,6-7,14,20,25H,1,4-5,9-10,12-13,15-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291314
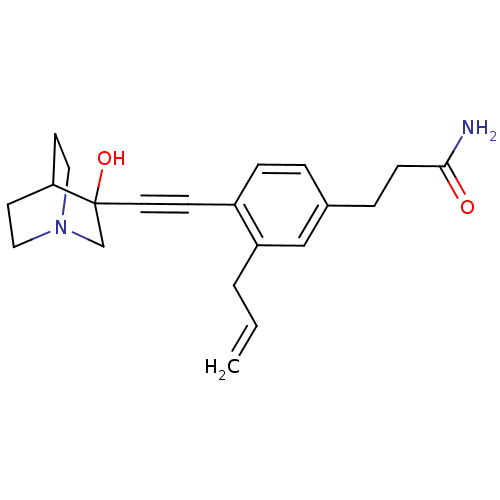
(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES NC(=O)CCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:10:11:16.15:18.19,12:11:16.15:18.19,(18.09,-2.89,;16.75,-2.12,;16.75,-.58,;15.43,-2.9,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.24,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.64,;3.26,-8.24,;3.14,-6.74,;4.77,-6.15,;4.96,-4.38,;5.36,-5.61,;10.09,-2.93,;8.76,-2.17,;7.43,-2.94,;6.1,-2.18,;11.43,-2.15,)| Show InChI InChI=1S/C21H26N2O2/c1-2-3-18-14-16(5-7-20(22)24)4-6-17(18)8-11-21(25)15-23-12-9-19(21)10-13-23/h2,4,6,14,19,25H,1,3,5,7,9-10,12-13,15H2,(H2,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291313
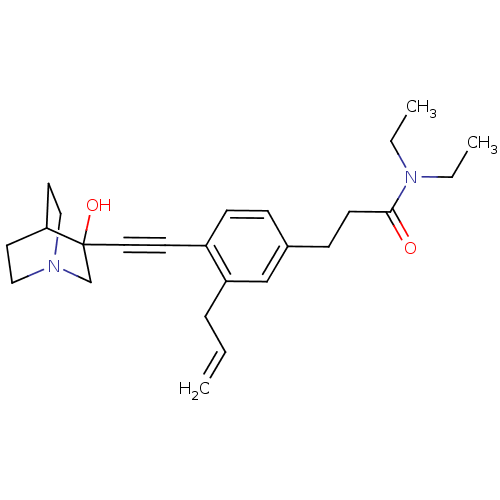
(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES CCN(CC)C(=O)CCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:14:15:20.19:22.23,16:15:20.19:22.23,(19.46,-5.19,;18.12,-4.43,;18.1,-2.89,;19.44,-2.11,;20.78,-2.86,;16.76,-2.12,;16.76,-.58,;15.44,-2.91,;14.1,-2.13,;12.77,-2.91,;12.77,-4.46,;11.45,-5.23,;10.12,-4.46,;8.78,-5.24,;7.45,-6.01,;6.19,-6.88,;7.67,-7.29,;6.4,-8.42,;4.96,-7.64,;3.26,-8.25,;3.14,-6.75,;4.78,-6.15,;4.96,-4.38,;5.37,-5.61,;10.09,-2.93,;8.77,-2.17,;7.44,-2.94,;6.1,-2.18,;11.43,-2.15,)| Show InChI InChI=1S/C25H34N2O2/c1-4-7-22-18-20(9-11-24(28)27(5-2)6-3)8-10-21(22)12-15-25(29)19-26-16-13-23(25)14-17-26/h4,8,10,18,23,29H,1,5-7,9,11,13-14,16-17,19H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291319
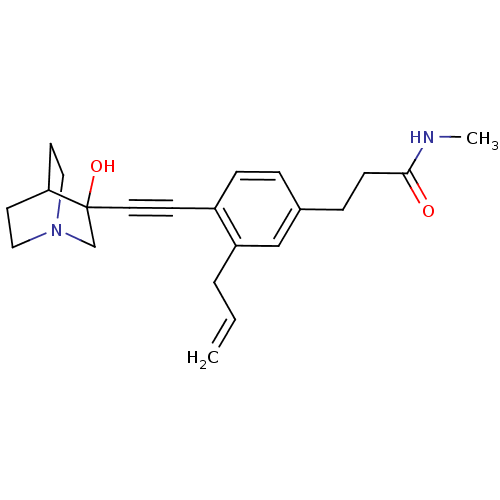
(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES CNC(=O)CCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:11:12:17.16:19.20,13:12:17.16:19.20,(19.42,-2.1,;18.08,-2.89,;16.75,-2.12,;16.75,-.58,;15.43,-2.9,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.23,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.63,;3.26,-8.24,;3.14,-6.74,;4.77,-6.14,;4.96,-4.38,;5.36,-5.6,;10.08,-2.93,;8.76,-2.17,;7.43,-2.94,;6.09,-2.18,;11.42,-2.15,)| Show InChI InChI=1S/C22H28N2O2/c1-3-4-19-15-17(6-8-21(25)23-2)5-7-18(19)9-12-22(26)16-24-13-10-20(22)11-14-24/h3,5,7,15,20,26H,1,4,6,8,10-11,13-14,16H2,2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291318
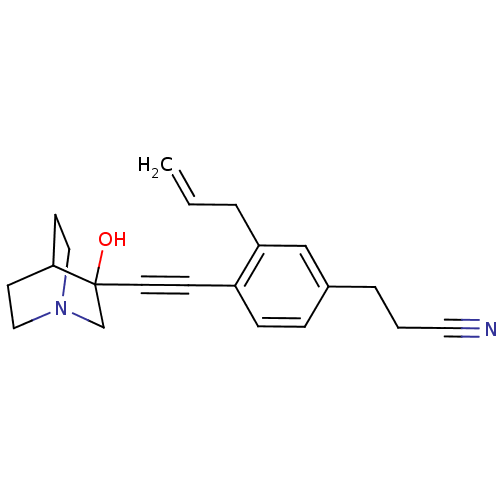
(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES OC1(CN2CCC1CC2)C#Cc1ccc(CCC#N)cc1CC=C |THB:9:1:5.4:7.8,0:1:5.4:7.8,(7.67,-7.29,;6.19,-6.88,;6.4,-8.42,;4.96,-7.64,;3.26,-8.25,;3.14,-6.75,;4.78,-6.15,;4.96,-4.38,;5.37,-5.61,;7.45,-6.01,;8.78,-5.24,;10.12,-4.46,;11.45,-5.23,;12.77,-4.46,;12.77,-2.91,;14.1,-2.13,;15.44,-2.91,;16.76,-2.12,;18.1,-1.35,;11.43,-2.15,;10.09,-2.93,;8.77,-2.17,;7.44,-2.94,;6.1,-2.18,)| Show InChI InChI=1S/C21H24N2O/c1-2-4-19-15-17(5-3-12-22)6-7-18(19)8-11-21(24)16-23-13-9-20(21)10-14-23/h2,6-7,15,20,24H,1,3-5,9-10,13-14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589205
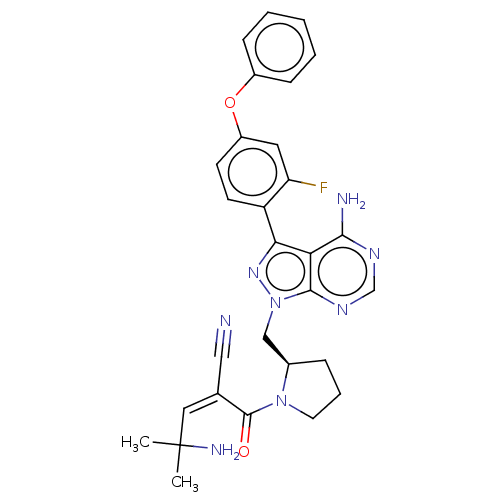
(CHEMBL5174021)Show SMILES CC(C)(N)\C=C(\C#N)C(=O)N1CCC[C@@H]1Cn1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589186
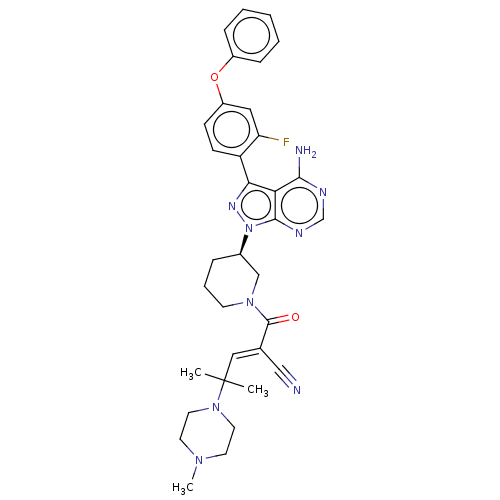
(CHEMBL5189379)Show SMILES CN1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589207
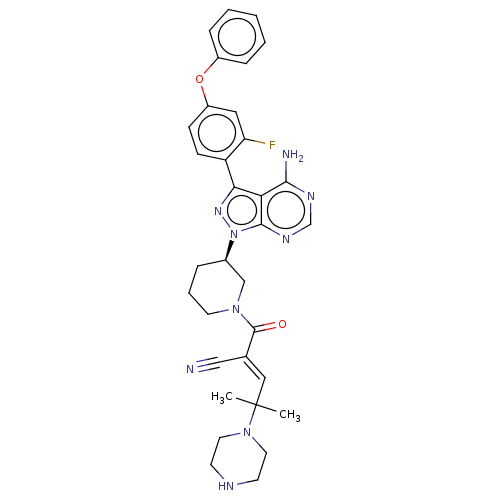
(CHEMBL5169419)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCNCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50589186

(CHEMBL5189379)Show SMILES CN1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50589186

(CHEMBL5189379)Show SMILES CN1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50589186

(CHEMBL5189379)Show SMILES CN1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589202
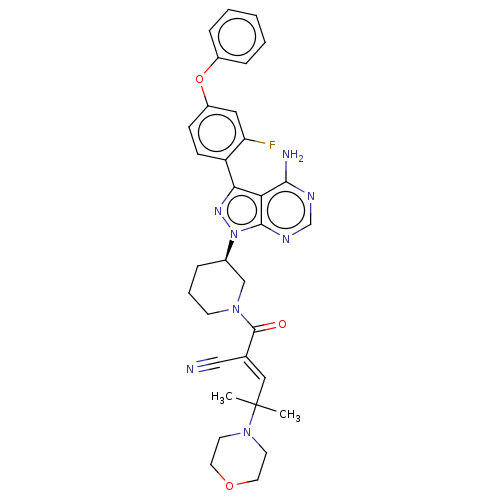
(CHEMBL5191633)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCOCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50557485
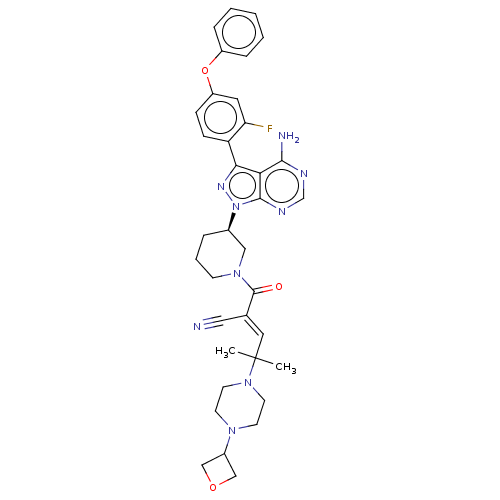
(Prn-1008 | Prn1008 | Rilzabrutinib)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCN(CC1)C1COC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589212
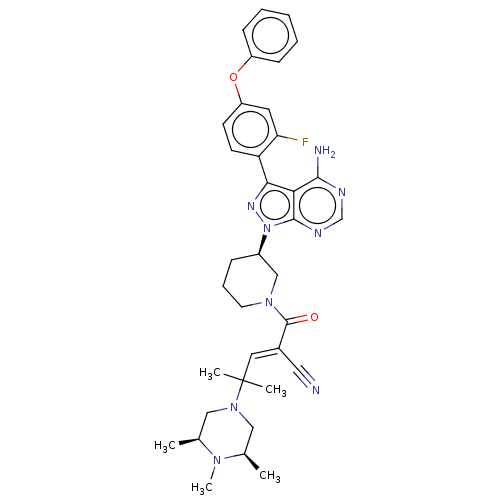
(CHEMBL5195816)Show SMILES C[C@H]1CN(C[C@@H](C)N1C)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589187
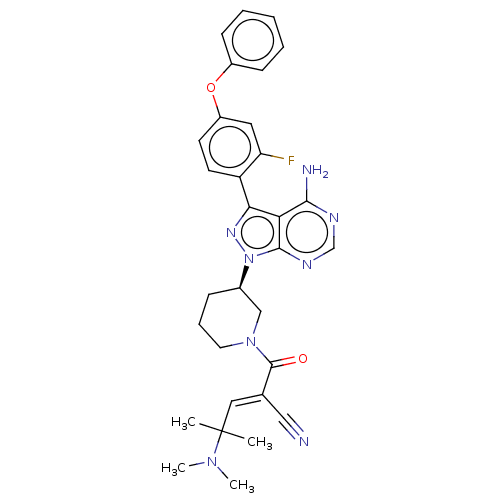
(CHEMBL5178314)Show SMILES CN(C)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589213
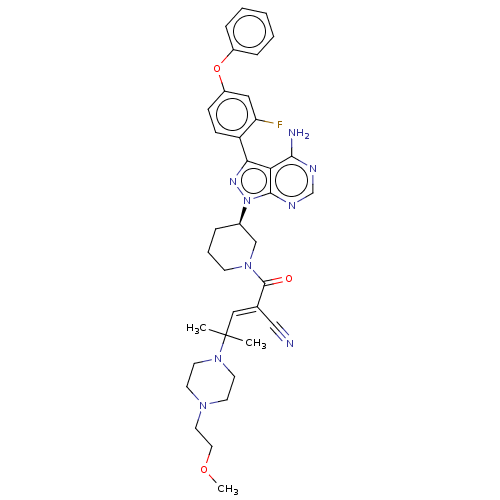
(CHEMBL3702850)Show SMILES COCCN1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50557485

(Prn-1008 | Prn1008 | Rilzabrutinib)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCN(CC1)C1COC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589200
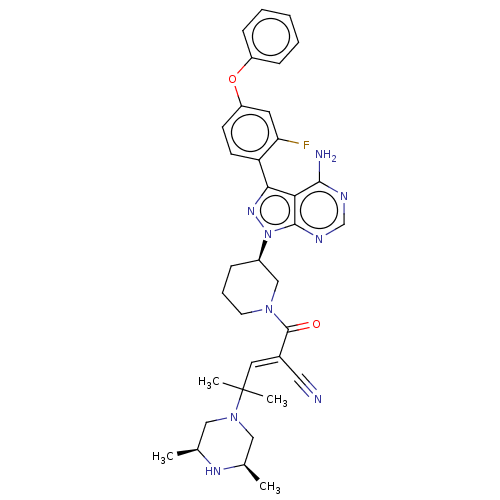
(CHEMBL5207901)Show SMILES C[C@H]1CN(C[C@@H](C)N1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM50557485

(Prn-1008 | Prn1008 | Rilzabrutinib)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCN(CC1)C1COC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM50589186

(CHEMBL5189379)Show SMILES CN1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589208

(CHEMBL3702851)Show SMILES CN1CCN(CC1=O)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589209
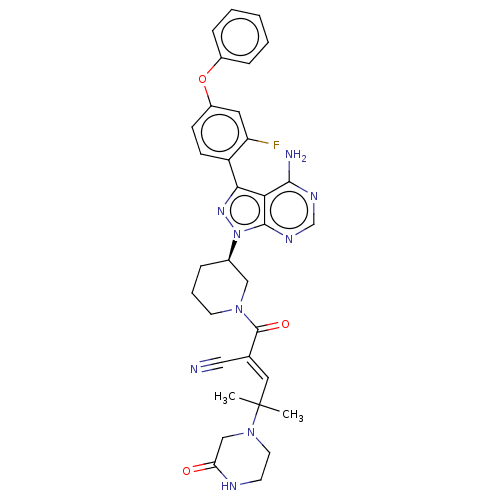
(CHEMBL3702858)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCNC(=O)C1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50557485

(Prn-1008 | Prn1008 | Rilzabrutinib)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCN(CC1)C1COC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50557485

(Prn-1008 | Prn1008 | Rilzabrutinib)Show SMILES CC(C)(\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCN(CC1)C1COC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50589191

(CHEMBL4114766)Show SMILES CC(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589191

(CHEMBL4114766)Show SMILES CC(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589201
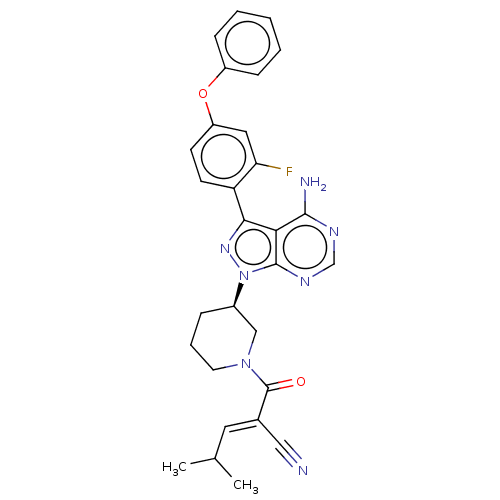
(CHEMBL5170583)Show SMILES CC(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50589191

(CHEMBL4114766)Show SMILES CC(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589211
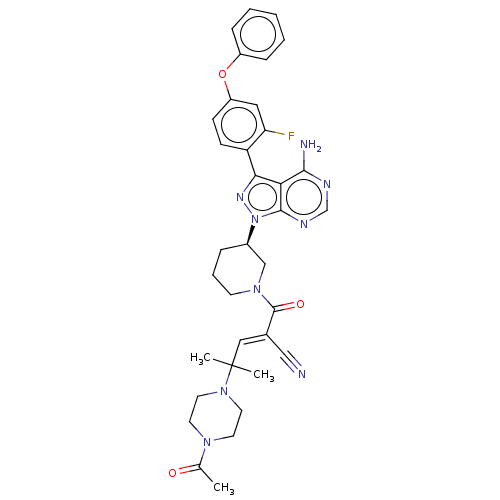
(CHEMBL3702855)Show SMILES CC(=O)N1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589192

(CHEMBL5204545)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3F)c12)[C@@H]1CCCN(C1)C(=O)C(=C\C1CC1)\C#N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589210

(CHEMBL3702856)Show SMILES COC(=O)N1CCN(CC1)C(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50589191

(CHEMBL4114766)Show SMILES CC(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589194
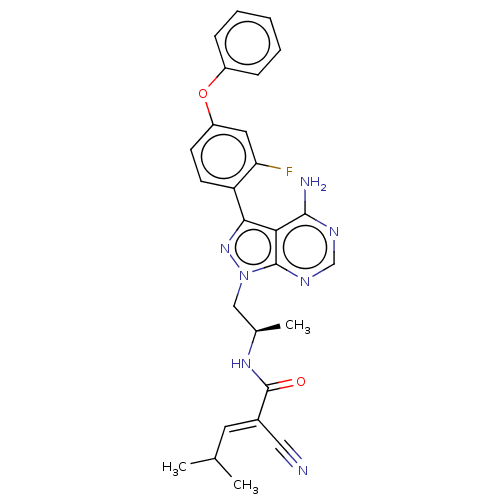
(CHEMBL4114703)Show SMILES CC(C)\C=C(/C#N)C(=O)N[C@H](C)Cn1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50052343
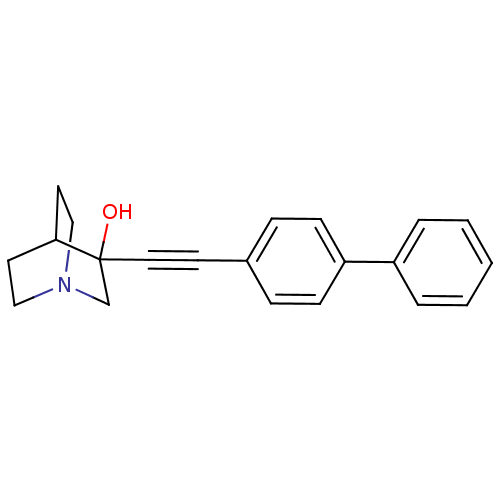
(3-Biphenyl-4-ylethynyl-1-aza-bicyclo[2.2.2]octan-3...)Show SMILES OC1(CN2CCC1CC2)C#Cc1ccc(cc1)-c1ccccc1 |THB:9:1:7.8:5.4,0:1:7.8:5.4,(8.8,-5.03,;8.54,-7.02,;8.83,-8.52,;7.36,-7.82,;5.68,-8.55,;5.47,-7.05,;7.08,-6.36,;7.08,-4.51,;7.64,-5.76,;10.08,-7.02,;10.91,-8.3,;11.75,-9.56,;13.23,-9.22,;14.27,-10.37,;13.78,-11.83,;12.28,-12.18,;11.24,-11.02,;14.83,-12.95,;16.33,-12.63,;17.37,-13.77,;16.92,-15.25,;15.39,-15.56,;14.38,-14.43,)| Show InChI InChI=1S/C21H21NO/c23-21(16-22-14-11-20(21)12-15-22)13-10-17-6-8-19(9-7-17)18-4-2-1-3-5-18/h1-9,20,23H,11-12,14-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition against rat microsomal squalene synthase (SS) |
J Med Chem 39: 2971-9 (1996)
Article DOI: 10.1021/jm950907l
BindingDB Entry DOI: 10.7270/Q24J0D7G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589188
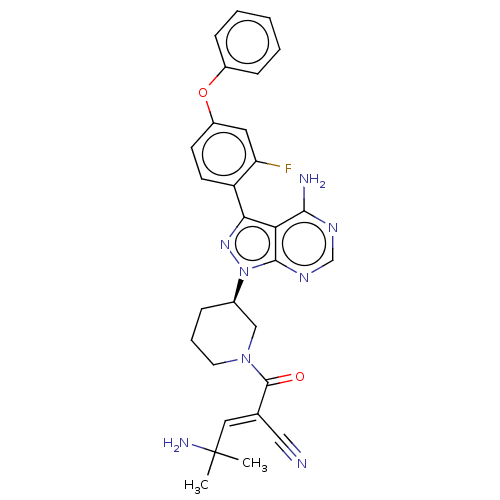
(CHEMBL5188669)Show SMILES CC(C)(N)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589189
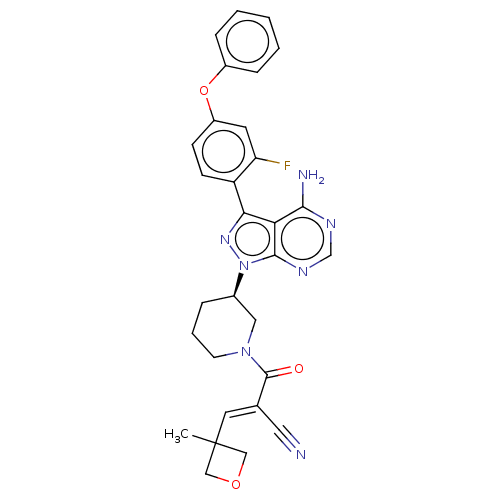
(CHEMBL3702827)Show SMILES CC1(COC1)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589193
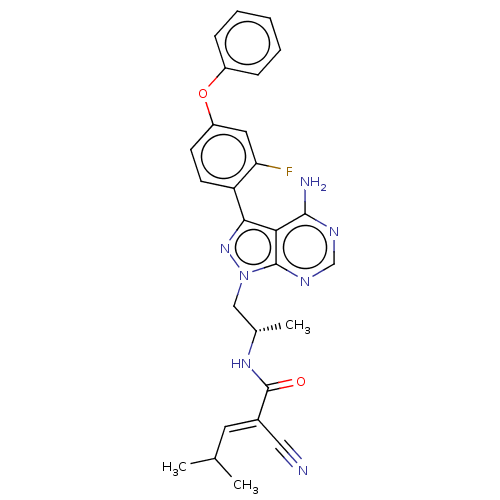
(CHEMBL3947620)Show SMILES [H][C@](C)(Cn1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)NC(=O)C(=C\C(C)C)\C#N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM50589191

(CHEMBL4114766)Show SMILES CC(C)(C)\C=C(/C#N)C(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589195
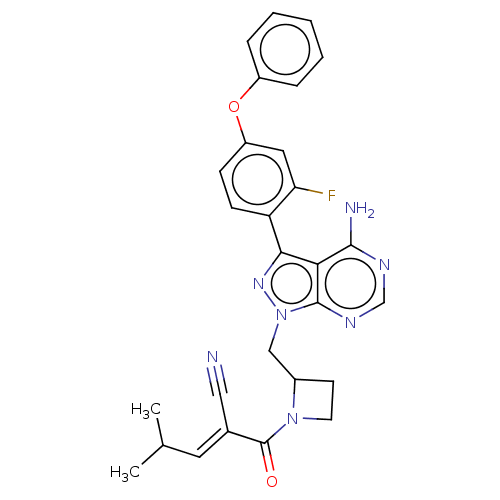
(CHEMBL3702838)Show SMILES CC(C)\C=C(/C#N)C(=O)N1CCC1Cn1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50052350
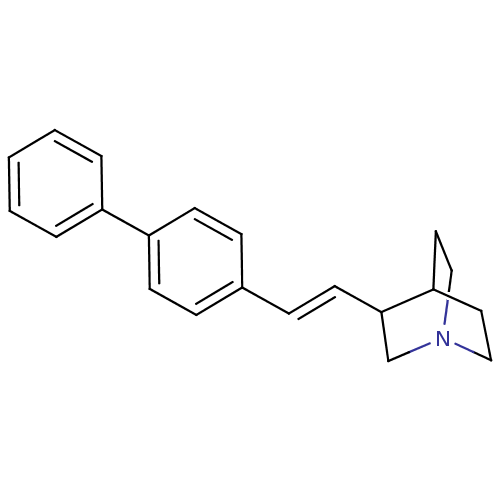
(3-((E)-2-Biphenyl-4-yl-vinyl)-1-aza-bicyclo[2.2.2]...)Show SMILES C1CN2CCC1C(C2)\C=C\c1ccc(cc1)-c1ccccc1 |THB:8:6:4.3:0.1,(9.53,-12.11,;9.74,-13.61,;11.4,-12.88,;11.68,-10.82,;11.12,-9.57,;11.12,-11.41,;12.6,-12.04,;12.88,-13.58,;13.78,-11.1,;15.21,-11.68,;16.43,-10.72,;16.11,-9.22,;17.24,-8.17,;18.7,-8.65,;19.05,-10.15,;17.9,-11.17,;19.85,-7.61,;19.53,-6.11,;20.65,-5.07,;22.14,-5.54,;22.47,-7.04,;21.32,-8.09,)| Show InChI InChI=1S/C21H23N/c1-2-4-18(5-3-1)19-9-6-17(7-10-19)8-11-21-16-22-14-12-20(21)13-15-22/h1-11,20-21H,12-16H2/b11-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition against rat microsomal squalene synthase (SS) |
J Med Chem 39: 2971-9 (1996)
Article DOI: 10.1021/jm950907l
BindingDB Entry DOI: 10.7270/Q24J0D7G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50369720
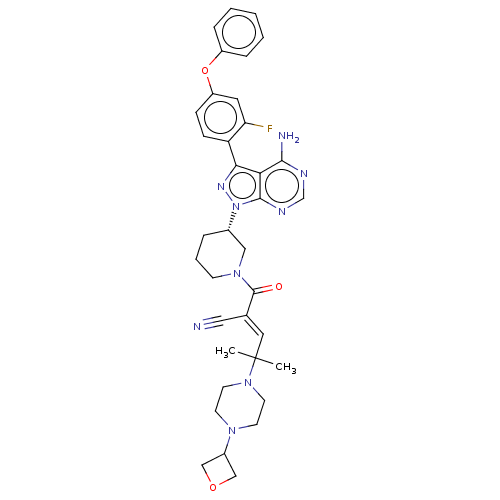
(CHEMBL3979355)Show SMILES [H][C@@]1(CCCN(C1)C(=O)C(=C\C(C)(C)N1CCN(CC1)C1COC1)\C#N)n1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| Show InChI InChI=1S/C36H40FN9O3/c1-36(2,45-15-13-43(14-16-45)26-21-48-22-26)18-24(19-38)35(47)44-12-6-7-25(20-44)46-34-31(33(39)40-23-41-34)32(42-46)29-11-10-28(17-30(29)37)49-27-8-4-3-5-9-27/h3-5,8-11,17-18,23,25-26H,6-7,12-16,20-22H2,1-2H3,(H2,39,40,41)/b24-18+/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50589198
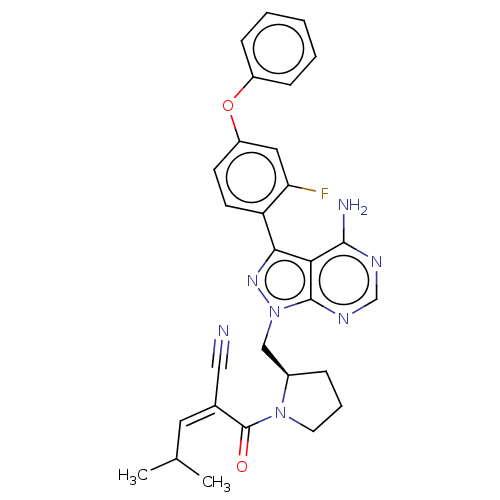
(CHEMBL5203733)Show SMILES CC(C)\C=C(\C#N)C(=O)N1CCC[C@@H]1Cn1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50589206
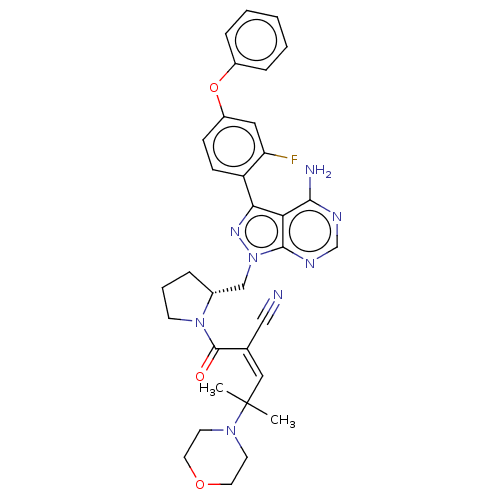
(CHEMBL5184084)Show SMILES CC(C)(\C=C(\C#N)C(=O)N1CCC[C@@H]1Cn1nc(-c2ccc(Oc3ccccc3)cc2F)c2c(N)ncnc12)N1CCOCC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01170
BindingDB Entry DOI: 10.7270/Q2W099WG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194411
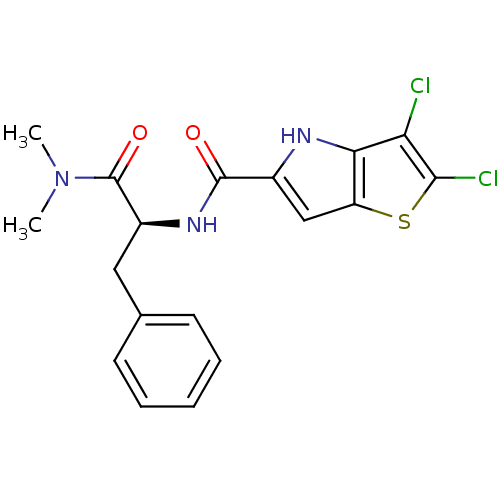
((S)-2,3-dichloro-N-(1-(dimethylamino)-1-oxo-3-phen...)Show SMILES CN(C)C(=O)[C@H](Cc1ccccc1)NC(=O)c1cc2sc(Cl)c(Cl)c2[nH]1 Show InChI InChI=1S/C18H17Cl2N3O2S/c1-23(2)18(25)12(8-10-6-4-3-5-7-10)22-17(24)11-9-13-15(21-11)14(19)16(20)26-13/h3-7,9,12,21H,8H2,1-2H3,(H,22,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human liver GPa by multienzyme coupled assay |
Bioorg Med Chem Lett 16: 5567-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.047
BindingDB Entry DOI: 10.7270/Q2KW5FP3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data