

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM81768 (BENZYLIC ACID | CAS_6581-06-2 | NSC_5311391 | Quin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation Curated by PDSP Ki Database | Biochem Pharmacol 45: 2352-4 (1993) Article DOI: 10.1016/0006-2952(93)90211-e BindingDB Entry DOI: 10.7270/Q2F76B2C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM81768 (BENZYLIC ACID | CAS_6581-06-2 | NSC_5311391 | Quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation Curated by PDSP Ki Database | Biochem Pharmacol 45: 2352-4 (1993) Article DOI: 10.1016/0006-2952(93)90211-e BindingDB Entry DOI: 10.7270/Q2F76B2C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM81768 (BENZYLIC ACID | CAS_6581-06-2 | NSC_5311391 | Quin...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation Curated by PDSP Ki Database | Biochem Pharmacol 45: 2352-4 (1993) Article DOI: 10.1016/0006-2952(93)90211-e BindingDB Entry DOI: 10.7270/Q2F76B2C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075807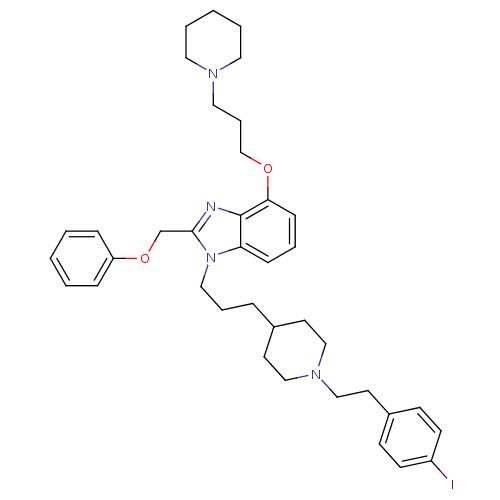 (1-(3-{1-[2-(4-Iodo-phenyl)-ethyl]-piperidin-4-yl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM81768 (BENZYLIC ACID | CAS_6581-06-2 | NSC_5311391 | Quin...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation Curated by PDSP Ki Database | Biochem Pharmacol 45: 2352-4 (1993) Article DOI: 10.1016/0006-2952(93)90211-e BindingDB Entry DOI: 10.7270/Q2F76B2C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM81768 (BENZYLIC ACID | CAS_6581-06-2 | NSC_5311391 | Quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation Curated by PDSP Ki Database | Biochem Pharmacol 45: 2352-4 (1993) Article DOI: 10.1016/0006-2952(93)90211-e BindingDB Entry DOI: 10.7270/Q2F76B2C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075796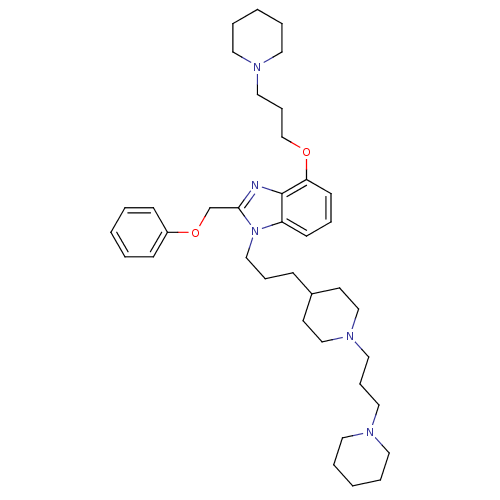 (2-Phenoxymethyl-4-(3-piperidin-1-yl-propoxy)-1-{3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50051751 (1-[3-(4-Isopropenyl-phenyl)-8-aza-bicyclo[3.2.1]oc...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-paroxetine from Serotonin transporter of rat frontal cortex membrane | J Med Chem 39: 2554-8 (1996) Article DOI: 10.1021/jm9600508 BindingDB Entry DOI: 10.7270/Q2V69HP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075811 (3-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-propo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50041293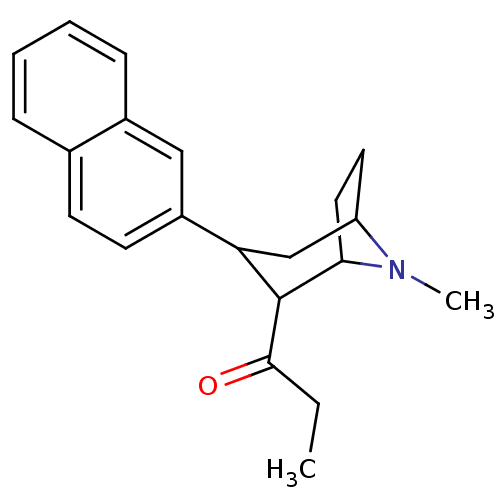 (1-(8-Methyl-3-naphthalen-2-yl-8-aza-bicyclo[3.2.1]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University Curated by ChEMBL | Assay Description Inhibition of [3H]paroxetine binding to serotonin transport sites in rat frontal cortex membranes. | J Med Chem 37: 1262-8 (1994) BindingDB Entry DOI: 10.7270/Q2TH8NBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50111887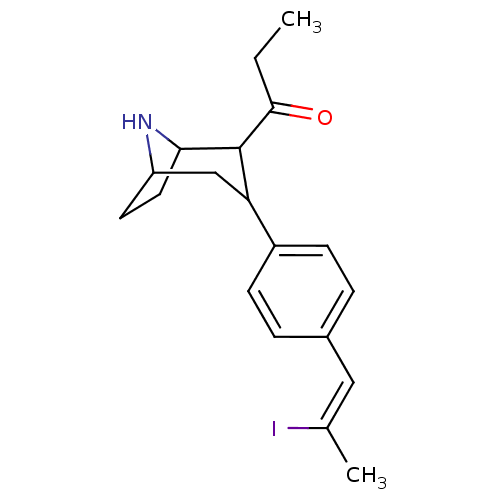 (1-{3-[4-((Z)-2-Iodo-propenyl)-phenyl]-8-aza-bicycl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Tested for the ability to displace [3H]- paroxetine binding in rat frontal cortex membrane against Serotonin transporter | Bioorg Med Chem Lett 12: 845-7 (2002) BindingDB Entry DOI: 10.7270/Q25M6515 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075803 (4-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-propo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50041293 (1-(8-Methyl-3-naphthalen-2-yl-8-aza-bicyclo[3.2.1]...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University Curated by PDSP Ki Database | J Pharmacol Exp Ther 293: 686-96 (2000) BindingDB Entry DOI: 10.7270/Q2H993QF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50499869 (CHEMBL3742140) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]Spiroperidol from cloned rat dopamine D3 receptor expressed in HEK293 cells by liquid scintillation counting analysis | J Med Chem 58: 9179-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b01031 BindingDB Entry DOI: 10.7270/Q2N019HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000041 ((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against human NK1 receptor was determined | Bioorg Med Chem Lett 5: 2671-2676 (1995) Article DOI: 10.1016/0960-894X(95)00481-8 BindingDB Entry DOI: 10.7270/Q2V40V5B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075812 (2-Phenoxymethyl-1-{3-[1-((Z)-3-phenyl-allyl)-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50307830 ((S)-6-(propyl(2-(4-(quinolin-4-yl)piperazin-1-yl)e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from cloned dopamine D3 receptor expressed in HEK cells | J Med Chem 53: 2114-25 (2010) Article DOI: 10.1021/jm901618d BindingDB Entry DOI: 10.7270/Q20K28QZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50303799 ((-)-(S)-6-(Propyl(2-(4-(quinolin-4-yl)piperazin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in HEK293 cells | J Med Chem 53: 1023-37 (2010) Article DOI: 10.1021/jm901184n BindingDB Entry DOI: 10.7270/Q29023VM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075809 (1-[3-(1-Phenethyl-piperidin-4-yl)-propyl]-2-phenox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50051753 (1-(3-Naphthalen-2-yl-8-aza-bicyclo[3.2.1]oct-2-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-paroxetine from Serotonin transporter of rat frontal cortex membrane | J Med Chem 39: 2554-8 (1996) Article DOI: 10.1021/jm9600508 BindingDB Entry DOI: 10.7270/Q2V69HP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50051753 (1-(3-Naphthalen-2-yl-8-aza-bicyclo[3.2.1]oct-2-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Affinity for the displacement of [3H]-paroxetine binding to serotonin transporter (SERT) in rat frontal cortex membranes | J Med Chem 44: 1509-15 (2001) BindingDB Entry DOI: 10.7270/Q2H1318R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.270 | -50.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Bioorg Med Chem Lett 18: 5567-70 (2008) Article DOI: 10.1016/j.bmcl.2008.09.002 BindingDB Entry DOI: 10.7270/Q2W0948B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.280 | -50.7 | n/a | n/a | 1 | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Biochem Biophys Res Commun 244: 1-4 (1998) Article DOI: 10.1006/bbrc.1998.8209 BindingDB Entry DOI: 10.7270/Q2930RFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075797 (1-{3-[1-(2-Cyclohexyl-ethyl)-piperidin-4-yl]-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.285 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075806 (1-{3-[1-(3-Methyl-butyl)-piperidin-4-yl]-propyl}-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18699 ((2R)-3-bromo-2-hydroxy-2-methyl-N-[4-nitro-3-(trif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee-Health Science Center Curated by ChEMBL | Assay Description Binding affinity against human androgen receptor (hAR) in competitive binding assay | J Med Chem 44: 1729-40 (2001) BindingDB Entry DOI: 10.7270/Q25M650Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057761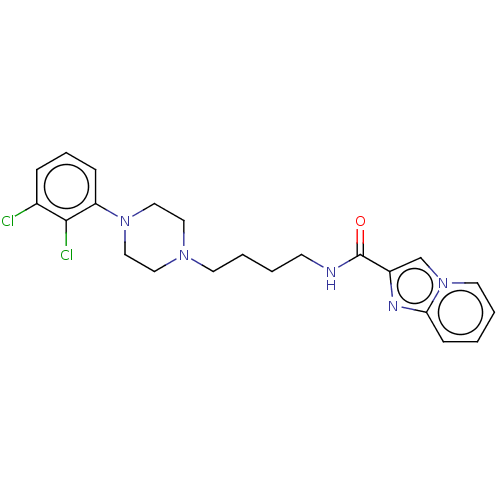 (CHEMBL3322994) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human D3R expressed in HEK293 cell membranes by gamma counting method | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18699 ((2R)-3-bromo-2-hydroxy-2-methyl-N-[4-nitro-3-(trif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18699 ((2R)-3-bromo-2-hydroxy-2-methyl-N-[4-nitro-3-(trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.300 | -50.5 | n/a | n/a | 500 | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Biochem Biophys Res Commun 244: 1-4 (1998) Article DOI: 10.1006/bbrc.1998.8209 BindingDB Entry DOI: 10.7270/Q2930RFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075802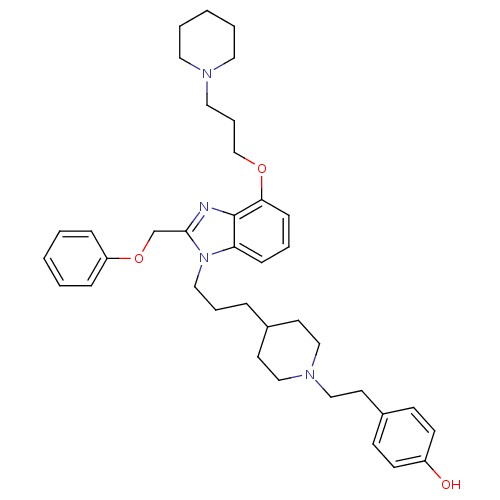 (4-[2-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50051757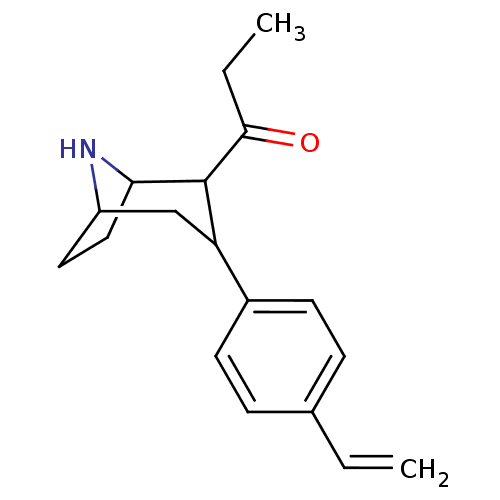 (1-[3-(4-Vinyl-phenyl)-8-aza-bicyclo[3.2.1]oct-2-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-paroxetine from Serotonin transporter of rat frontal cortex membrane | J Med Chem 39: 2554-8 (1996) Article DOI: 10.1021/jm9600508 BindingDB Entry DOI: 10.7270/Q2V69HP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin receptor subtype-3 (Homo sapiens (Human)) | BDBM50275902 (CHEMBL525577 | D-Phe-Gln-Trp-Ala-Val-b-Ala-His-Phe...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to BRS-3 receptor (unknown origin) | Bioorg Med Chem Lett 18: 5451-5 (2008) Article DOI: 10.1016/j.bmcl.2008.09.033 BindingDB Entry DOI: 10.7270/Q2H9952R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075799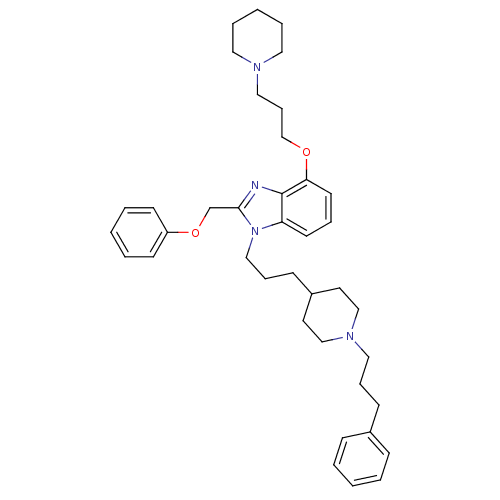 (2-Phenoxymethyl-1-{3-[1-(3-phenyl-propyl)-piperidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.361 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50118812 ((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in HEK293 cells by radioligand binding assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00685 BindingDB Entry DOI: 10.7270/Q2930Z2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50099228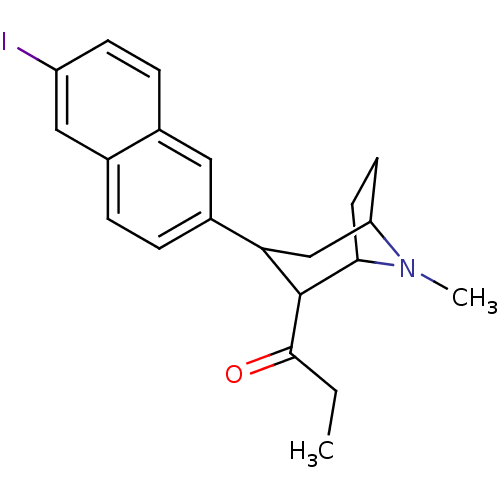 (1-[3-(6-Iodo-naphthalen-2-yl)-8-methyl-8-aza-bicyc...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Affinity for the displacement of [3H]-paroxetine binding to serotonin transporter (SERT) in rat frontal cortex membranes | J Med Chem 44: 1509-15 (2001) BindingDB Entry DOI: 10.7270/Q2H1318R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50099227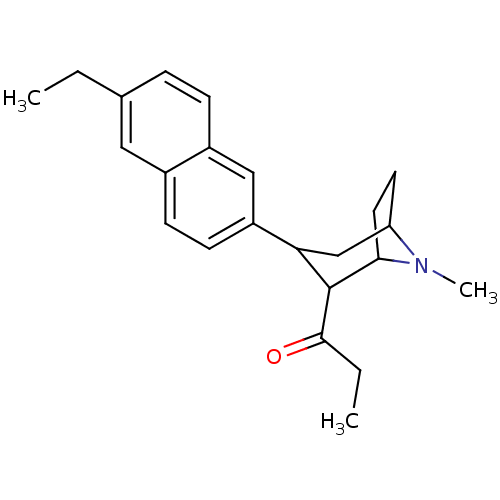 (1-[3-(6-Ethyl-naphthalen-2-yl)-8-methyl-8-aza-bicy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Affinity for the displacement of [3H]-paroxetine binding to serotonin transporter (SERT) in rat frontal cortex membranes | J Med Chem 44: 1509-15 (2001) BindingDB Entry DOI: 10.7270/Q2H1318R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50041293 (1-(8-Methyl-3-naphthalen-2-yl-8-aza-bicyclo[3.2.1]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-paroxetine from Serotonin transporter of rat frontal cortex membrane | J Med Chem 39: 2554-8 (1996) Article DOI: 10.1021/jm9600508 BindingDB Entry DOI: 10.7270/Q2V69HP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075798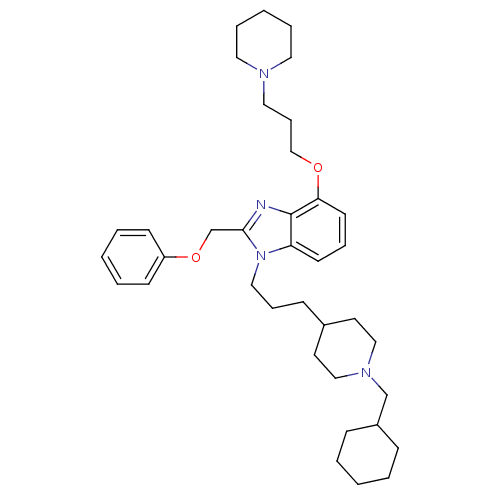 (1-[3-(1-Cyclohexylmethyl-piperidin-4-yl)-propyl]-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.393 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50041293 (1-(8-Methyl-3-naphthalen-2-yl-8-aza-bicyclo[3.2.1]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.394 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University Curated by ChEMBL | Assay Description Inhibition of [3H]paroxetine binding to serotonin transport sites in rat frontal cortex membranes. | J Med Chem 37: 1262-8 (1994) BindingDB Entry DOI: 10.7270/Q2TH8NBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50081533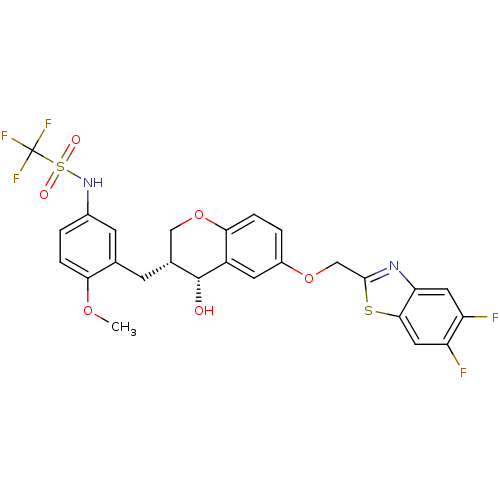 (CHEMBL96224 | CP-199331 | N-{3-[(3R,4R)-6-(5,6-Dif...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the binding of Cysteinyl leukotriene receptor 1 to guinea pig lung membranes | Bioorg Med Chem Lett 9: 2773-8 (1999) BindingDB Entry DOI: 10.7270/Q2MP52G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50303799 ((-)-(S)-6-(Propyl(2-(4-(quinolin-4-yl)piperazin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in HEK293 cells | J Med Chem 53: 1023-37 (2010) Article DOI: 10.1021/jm901184n BindingDB Entry DOI: 10.7270/Q29023VM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM50049736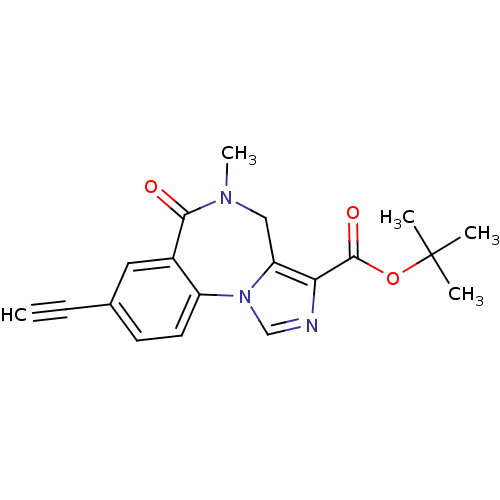 (8-Ethynyl-5-methyl-6-oxo-5,6-dihydro-4H-2,5,10b-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Moltech Corporation Curated by ChEMBL | Assay Description Binding affinity to GABAA alpha-5-beta-3-gamma-2 receptor | J Med Chem 51: 3788-803 (2008) Article DOI: 10.1021/jm701433b BindingDB Entry DOI: 10.7270/Q2FQ9XH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM22167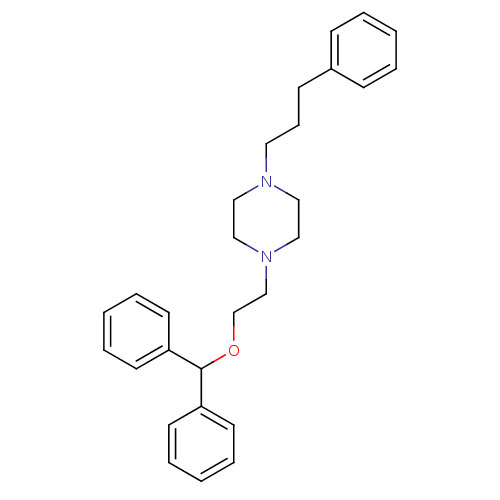 (1-[2-(diphenylmethoxy)ethyl]-4-(3-phenylpropyl)pip...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University Curated by PDSP Ki Database | J Pharmacol Exp Ther 293: 686-96 (2000) BindingDB Entry DOI: 10.7270/Q2H993QF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM22165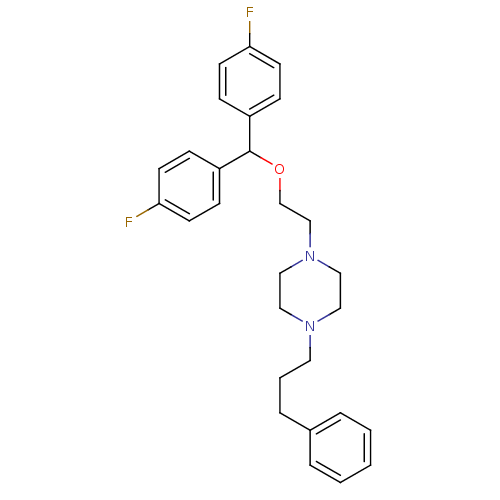 (1-{2-[bis(4-fluorophenyl)methoxy]ethyl}-4-(3-pheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University Curated by PDSP Ki Database | J Pharmacol Exp Ther 293: 686-96 (2000) BindingDB Entry DOI: 10.7270/Q2H993QF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.430 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee Health Science Center | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Bioorg Med Chem 14: 6525-38 (2006) Article DOI: 10.1016/j.bmc.2006.06.019 BindingDB Entry DOI: 10.7270/Q2WQ022N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50303796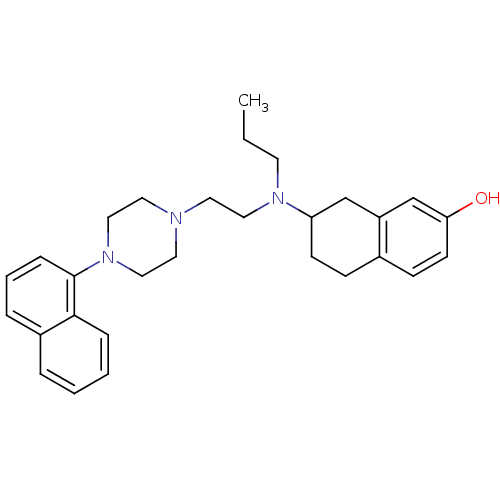 (7-((2-(4-(Naphthalen-1-yl)-piperazin-1-yl)ethyl)(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.435 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in HEK293 cells | J Med Chem 53: 1023-37 (2010) Article DOI: 10.1021/jm901184n BindingDB Entry DOI: 10.7270/Q29023VM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50303795 (7-((2-(4-(Isoquinolin-1-yl)piperazin-1-yl)ethyl)(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.441 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in HEK293 cells | J Med Chem 53: 1023-37 (2010) Article DOI: 10.1021/jm901184n BindingDB Entry DOI: 10.7270/Q29023VM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215713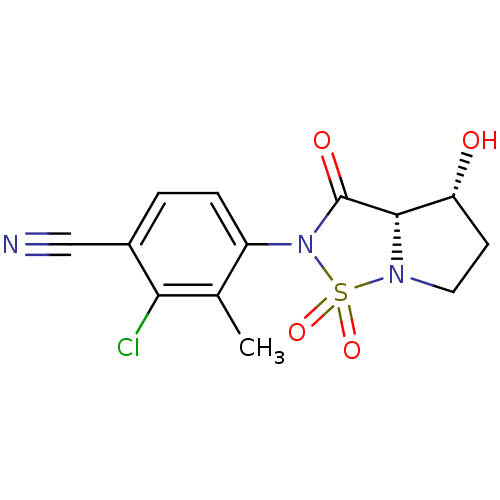 (2-Chloro-4-((3aS,4R)-4-hydroxy-1,1,3-trioxo-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50099223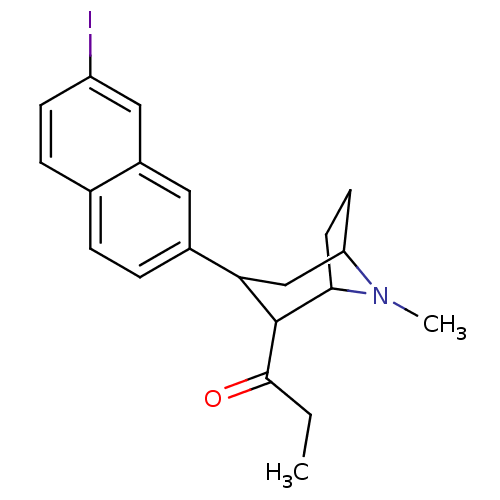 (1-[3-(7-Iodo-naphthalen-2-yl)-8-methyl-8-aza-bicyc...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Affinity for the displacement of [3H]-paroxetine binding to serotonin transporter (SERT) in rat frontal cortex membranes | J Med Chem 44: 1509-15 (2001) BindingDB Entry DOI: 10.7270/Q2H1318R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR2 (Homo sapiens (Human)) | BDBM312140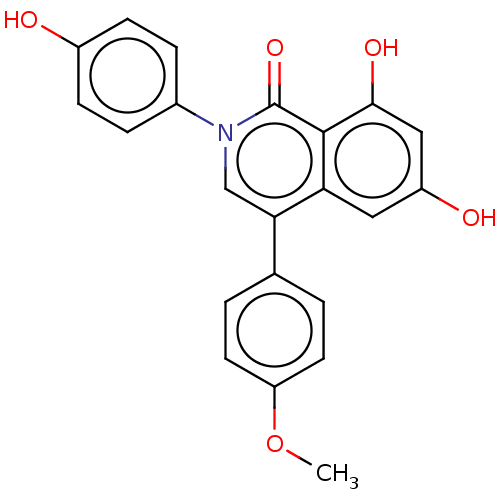 (6,8-dihydroxy-2-(4-hydroxyphenyl)-4-(4-methoxyphen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPARTMENT OF VETERANS AFFAIRS US Patent | Assay Description Estrogen receptor (ERβ) binding affinity of the NRBAs was also determined using an in vitro competitive radioligand-binding assay with [3H]-estr... | US Patent US9604931 (2017) BindingDB Entry DOI: 10.7270/Q23N25FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 15532 total ) | Next | Last >> |