| Reaction Details |
|---|
 | Report a problem with these data |
| Target | cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A |
|---|
| Ligand | BDBM50219268 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_457131 (CHEMBL940740) |
|---|
| IC50 | 130±n/a nM |
|---|
| Citation |  Kuang, R; Shue, HJ; Blythin, DJ; Shih, NY; Gu, D; Chen, X; Schwerdt, J; Lin, L; Ting, PC; Zhu, X; Aslanian, R; Piwinski, JJ; Xiao, L; Prelusky, D; Wu, P; Zhang, J; Zhang, X; Celly, CS; Minnicozzi, M; Billah, M; Wang, P Discovery of a highly potent series of oxazole-based phosphodiesterase 4 inhibitors. Bioorg Med Chem Lett17:5150-4 (2007) [PubMed] Article Kuang, R; Shue, HJ; Blythin, DJ; Shih, NY; Gu, D; Chen, X; Schwerdt, J; Lin, L; Ting, PC; Zhu, X; Aslanian, R; Piwinski, JJ; Xiao, L; Prelusky, D; Wu, P; Zhang, J; Zhang, X; Celly, CS; Minnicozzi, M; Billah, M; Wang, P Discovery of a highly potent series of oxazole-based phosphodiesterase 4 inhibitors. Bioorg Med Chem Lett17:5150-4 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A |
|---|
| Name: | cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A |
|---|
| Synonyms: | 3',5'-cyclic phosphodiesterase | 3.1.4.17 | PDE10A | PDE10_HUMAN | Phosphodiesterase 10 (PDE10) | Phosphodiesterase 10A |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 88412.52 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q9Y233 |
|---|
| Residue: | 1055 |
|---|
| Sequence: | MASLEEPLAPRPQGPLPAAGDEPGCGPGKLRPEPRLSAAGGGSAAGPGPAPEWPGRGRAE
RAAPPRPPLSSAGRPSPAGGPGALSARGGGCGWVAARAPLALAFSSRVPSSSPSFFYFWP
PPPPPPPSFLPSSSAFHLPVRLPGREGAAAAAAAGGGGDAGGGGGGGQEAAPLSVPTSSS
HRGGGGSGGGRRRLFLSPALQGLLLPARAGPRPPPPPRLPLGQAARRAGSPGFPGAGPGG
GGQTPRRPQGASFALAAAAALLFGSDMEDGPSNNASCFRRLTECFLSPSLTDEKVKAYLS
LHPQVLDEFVSESVSAETVEKWLKRKNNKSEDESAPKEVSRYQDTNMQGVVYELNSYIEQ
RLDTGGDNQLLLYELSSIIKIATKADGFALYFLGECNNSLCIFTPPGIKEGKPRLIPAGP
ITQGTTVSAYVAKSRKTLLVEDILGDERFPRGTGLESGTRIQSVLCLPIVTAIGDLIGIL
ELYRHWGKEAFCLSHQEVATANLAWASVAIHQVQVCRGLAKQTELNDFLLDVSKTYFDNI
VAIDSLLEHIMIYAKNLVNADRCALFQVDHKNKELYSDLFDIGEEKEGKPVFKKTKEIRF
SIEKGIAGQVARTGEVLNIPDAYADPRFNREVDLYTGYTTRNILCMPIVSRGSVIGVVQM
VNKISGSAFSKTDENNFKMFAVFCALALHCANMYHRIRHSECIYRVTMEKLSYHSICTSE
EWQGLMQFTLPVRLCKEIELFHFDIGPFENMWPGIFVYMVHRSCGTSCFELEKLCRFIMS
VKKNYRRVPYHNWKHAVTVAHCMYAILQNNHTLFTDLERKGLLIACLCHDLDHRGFSNSY
LQKFDHPLAALYSTSTMEQHHFSQTVSILQLEGHNIFSTLSSSEYEQVLEIIRKAIIATD
LALYFGNRKQLEEMYQTGSLNLNNQSHRDRVIGLMMTACDLCSVTKLWPVTKLTANDIYA
EFWAEGDEMKKLGIQPIPMMDRDKKDEVPQGQLGFYNAVAIPCYTTLTQILPPTEPLLKA
CRDNLSQWEKVIRGEETATWISSPSVAQKAAASED
|
|
|
|---|
| BDBM50219268 |
|---|
| n/a |
|---|
| Name | BDBM50219268 |
|---|
| Synonyms: | CHEMBL399298 | tert-butyl (R)-1-(5-(aminomethyl)-2-(8-methoxy-2-(trifluoromethyl)quinolin-5-yl)oxazole-4-carbonyl)pyrrolidin-3-ylcarbamate |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H28F3N5O5 |
|---|
| Mol. Mass. | 535.5155 |
|---|
| SMILES | COc1ccc(-c2nc(C(=O)N3CC[C@H](C3)NC(=O)OC(C)(C)C)c(CN)o2)c2ccc(nc12)C(F)(F)F |
|---|
| Structure | 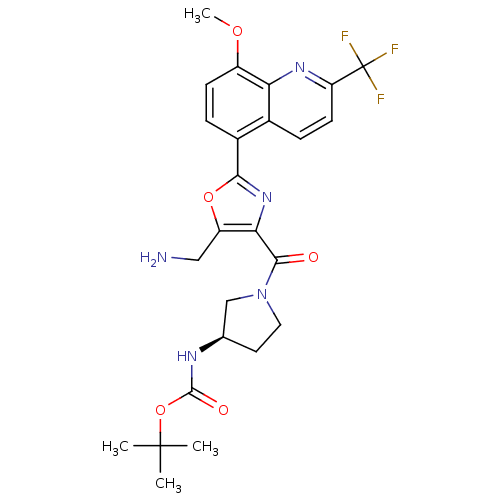
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kuang, R; Shue, HJ; Blythin, DJ; Shih, NY; Gu, D; Chen, X; Schwerdt, J; Lin, L; Ting, PC; Zhu, X; Aslanian, R; Piwinski, JJ; Xiao, L; Prelusky, D; Wu, P; Zhang, J; Zhang, X; Celly, CS; Minnicozzi, M; Billah, M; Wang, P Discovery of a highly potent series of oxazole-based phosphodiesterase 4 inhibitors. Bioorg Med Chem Lett17:5150-4 (2007) [PubMed] Article
Kuang, R; Shue, HJ; Blythin, DJ; Shih, NY; Gu, D; Chen, X; Schwerdt, J; Lin, L; Ting, PC; Zhu, X; Aslanian, R; Piwinski, JJ; Xiao, L; Prelusky, D; Wu, P; Zhang, J; Zhang, X; Celly, CS; Minnicozzi, M; Billah, M; Wang, P Discovery of a highly potent series of oxazole-based phosphodiesterase 4 inhibitors. Bioorg Med Chem Lett17:5150-4 (2007) [PubMed] Article