

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100528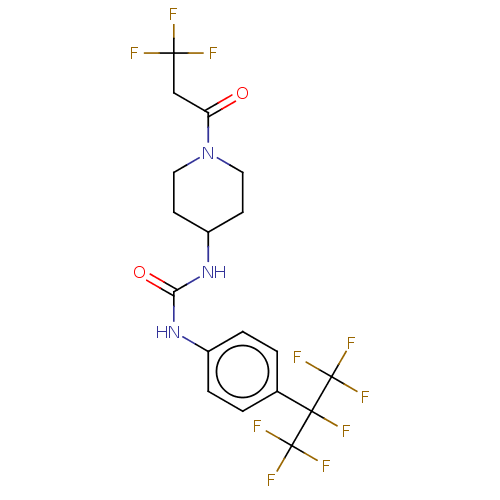 (CHEMBL3327081) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100535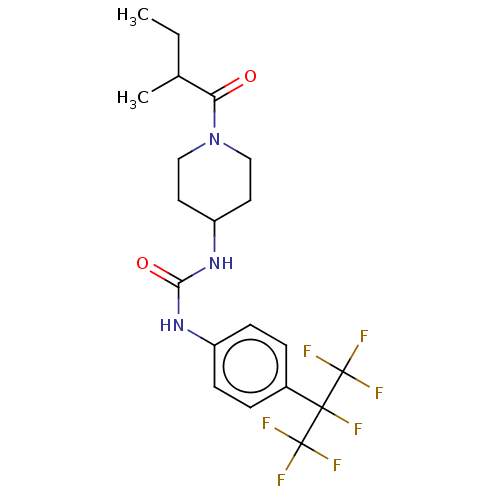 (CHEMBL3327073) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat forebrain nicotinic acetylcholine receptor using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat Nicotinic acetylcholine receptor alpha3-beta4 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat nicotinic acetylcholine receptor alpha2-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat Nicotinic acetylcholine receptor alpha3-beta4 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50143281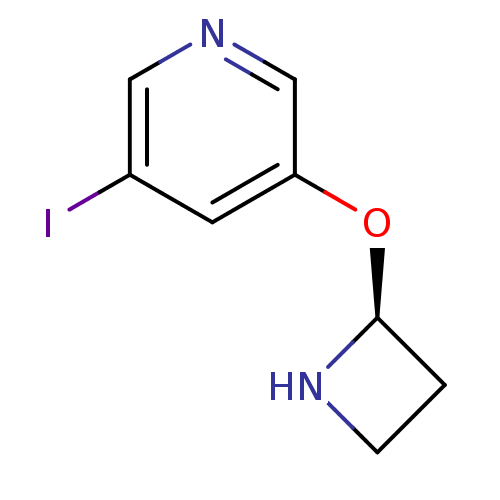 (3-((S)-Azetidin-2-yloxy)-5-iodo-pyridine | CHEMBL5...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat nicotinic acetylcholine receptor alpha2-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50143281 (3-((S)-Azetidin-2-yloxy)-5-iodo-pyridine | CHEMBL5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat Nicotinic acetylcholine receptor alpha3-beta4 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat nicotinic acetylcholine receptor alpha2-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50049750 ((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat nicotinic acetylcholine receptor alpha2-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049750 ((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against Nicotinic acetylcholine receptor using [3H]-(-)-nicotine radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100521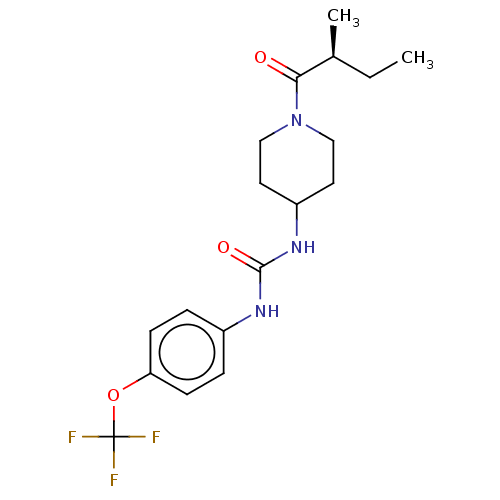 (CHEMBL3327078 | US10377744, Compound No. 2696) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100519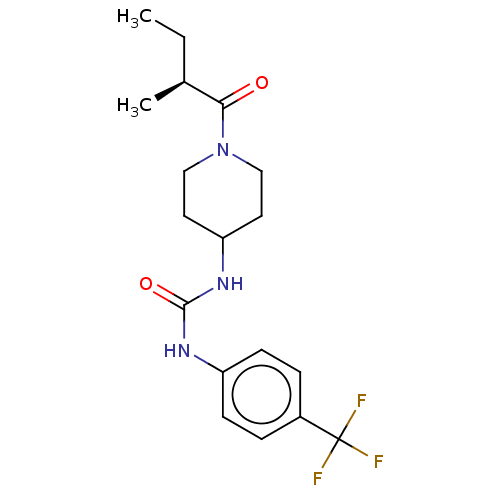 (CHEMBL3327067 | US10377744, Compound No. 2391) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100531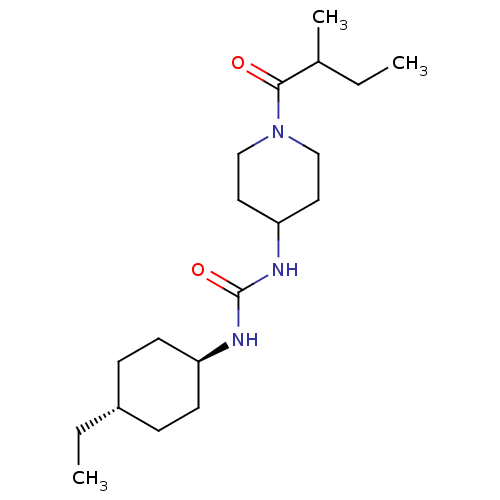 (CHEMBL3325465) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50049750 ((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat Nicotinic acetylcholine receptor alpha3-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100520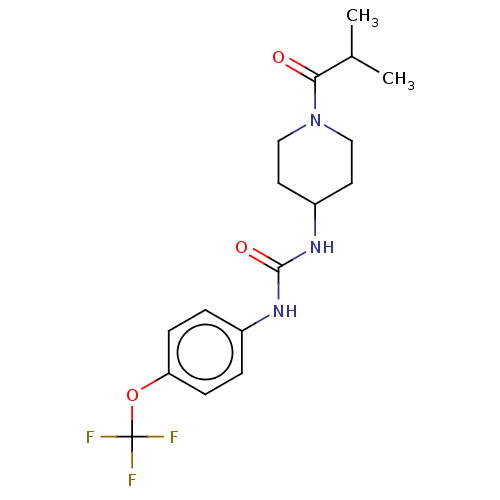 (CHEMBL3327077 | US10377744, Compound No. 2422 | US...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100534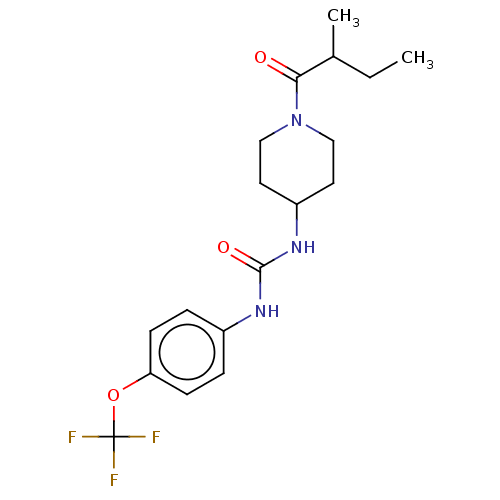 (CHEMBL3327074) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100541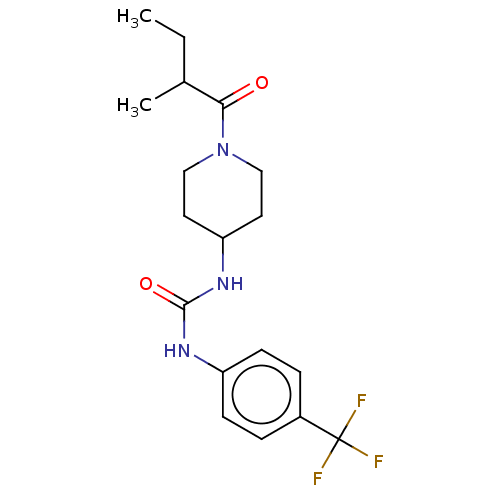 (CHEMBL3327066) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50143281 (3-((S)-Azetidin-2-yloxy)-5-iodo-pyridine | CHEMBL5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat nicotinic acetylcholine receptor alpha2-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100539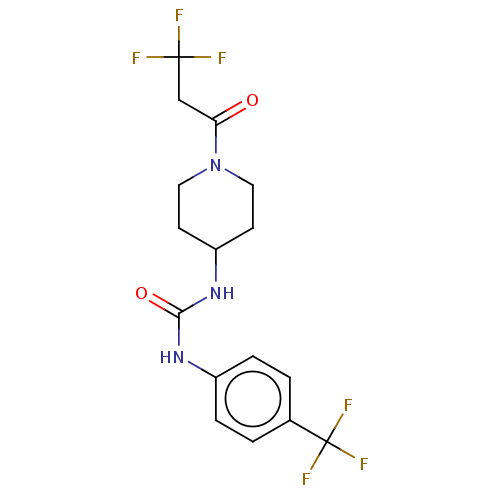 (CHEMBL3327069) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100522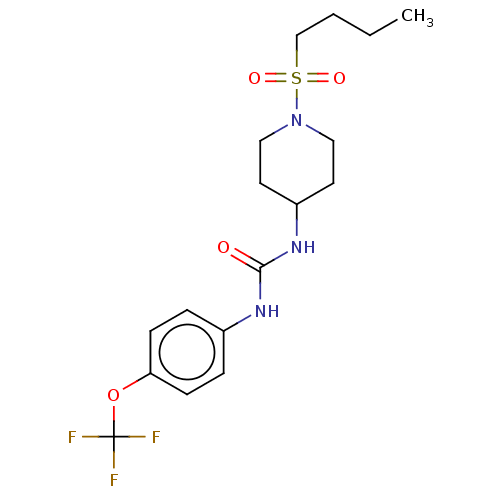 (CHEMBL3327087) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat Nicotinic acetylcholine receptor alpha3-beta2 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta4 expressed in HEK293 cells using [3H]EB as radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100542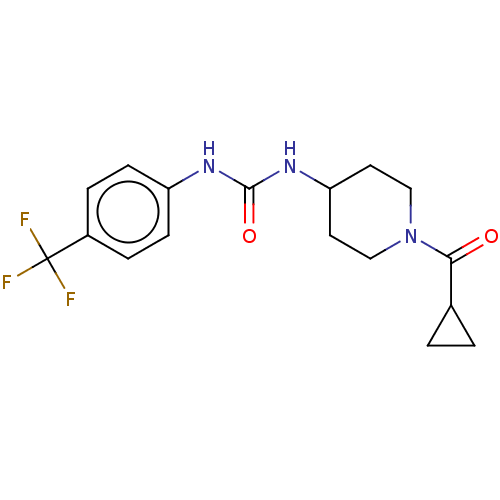 (CHEMBL3327065) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100540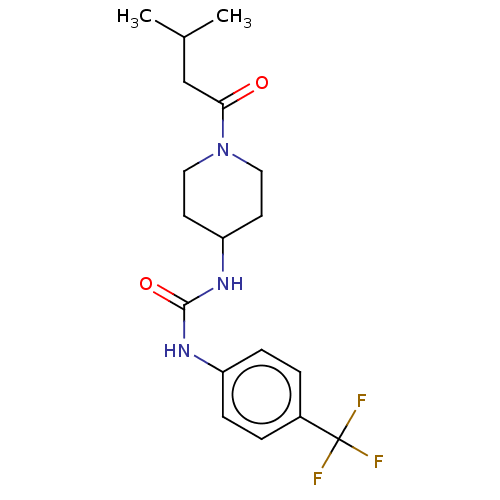 (CHEMBL3327068) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50327846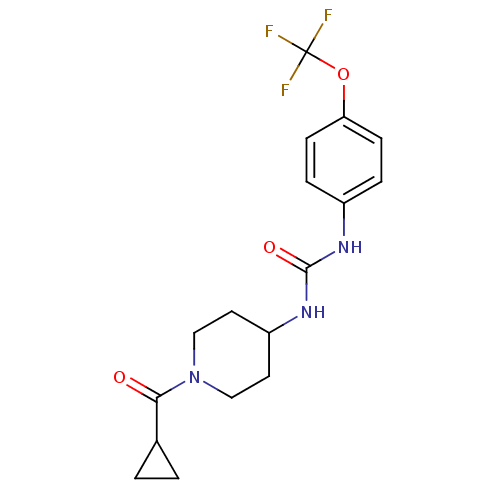 (1-(1-(Cyclopropanecarbonyl)piperidin-4-yl)-3-(4-(t...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50260067 (CHEMBL4078267 | US9957263, Example 78) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Biology Program, Department of Pharmacology and Toxicology,?Department of Internal Medicine,žSealy Center for Molecular Medicine,?Institute for Translational Sciences, University of Texas Me Curated by ChEMBL | Assay Description Inhibition of His-tagged BRD4 bromodomain 2 (E352 to E168 residues) (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 60: 4533-4558 (2017) Article DOI: 10.1021/acs.jmedchem.6b01761 BindingDB Entry DOI: 10.7270/Q27P91VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50260083 (CHEMBL4104956) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Biology Program, Department of Pharmacology and Toxicology,?Department of Internal Medicine,žSealy Center for Molecular Medicine,?Institute for Translational Sciences, University of Texas Me Curated by ChEMBL | Assay Description Inhibition of His-tagged BRD4 bromodomain 2 (E352 to E168 residues) (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 60: 4533-4558 (2017) Article DOI: 10.1021/acs.jmedchem.6b01761 BindingDB Entry DOI: 10.7270/Q27P91VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100518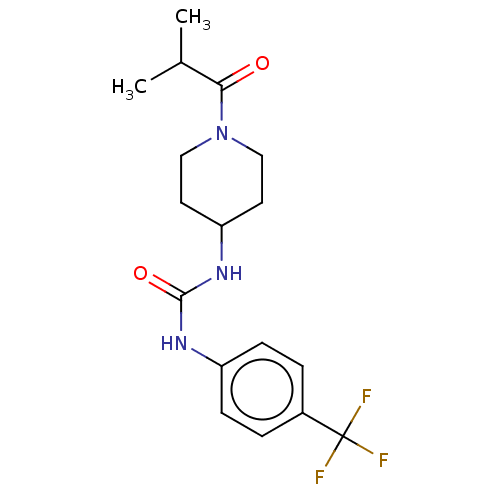 (CHEMBL3327064 | US10377744, Compound No. 2389) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50260067 (CHEMBL4078267 | US9957263, Example 78) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Biology Program, Department of Pharmacology and Toxicology,?Department of Internal Medicine,žSealy Center for Molecular Medicine,?Institute for Translational Sciences, University of Texas Me Curated by ChEMBL | Assay Description Inhibition of His-tagged BRD4 bromodomain 1 (K57 to E168 residues) (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 60: 4533-4558 (2017) Article DOI: 10.1021/acs.jmedchem.6b01761 BindingDB Entry DOI: 10.7270/Q27P91VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50327809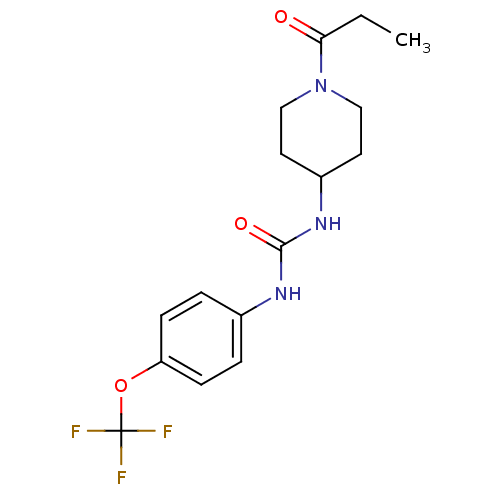 (1-(1-Propionylpiperidin-4-yl)-3-(4-(trifluorometho...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50526945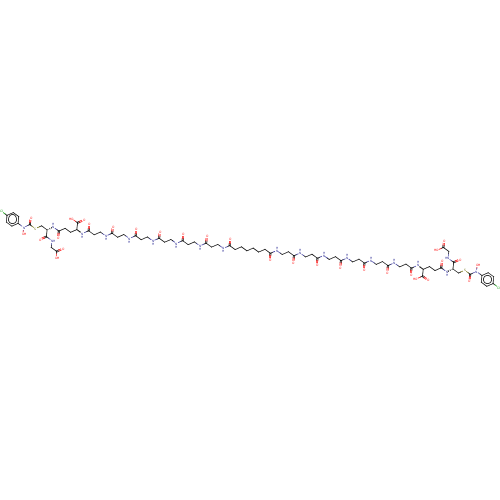 (CHEMBL4473806) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate by Michaelis-Menten analysis | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100523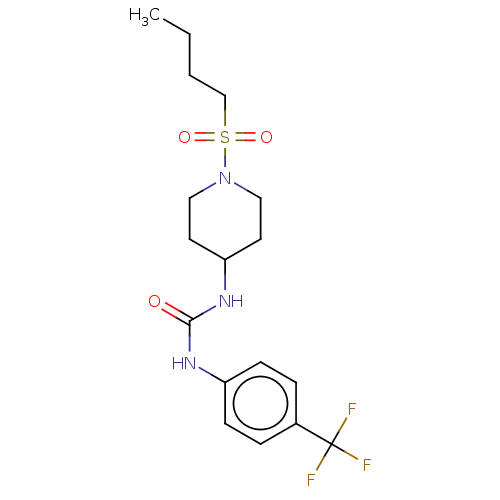 (CHEMBL3327086) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100532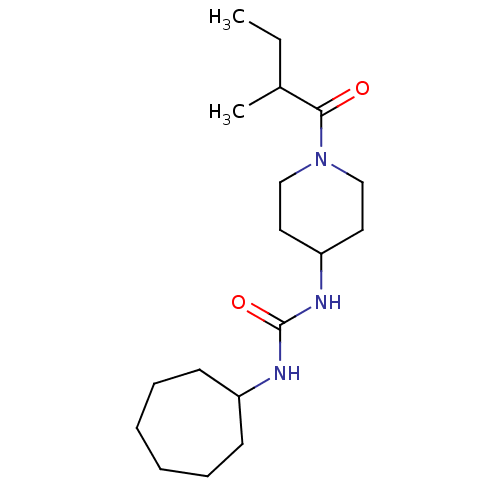 (CHEMBL3327076) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50526943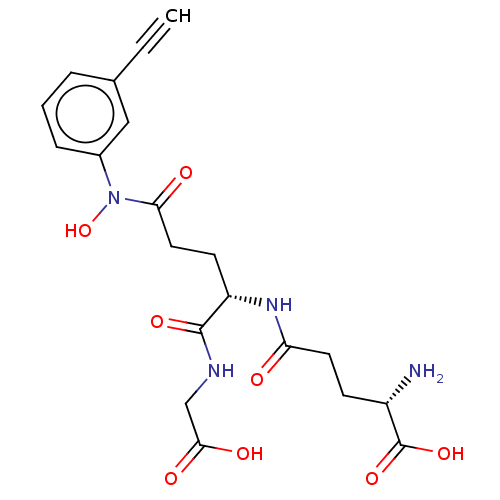 (CHEMBL4436073) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50260084 (CHEMBL4096313 | US9957263, Example 110) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Biology Program, Department of Pharmacology and Toxicology,?Department of Internal Medicine,žSealy Center for Molecular Medicine,?Institute for Translational Sciences, University of Texas Me Curated by ChEMBL | Assay Description Inhibition of His-tagged BRD4 bromodomain 1 (K57 to E168 residues) (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 60: 4533-4558 (2017) Article DOI: 10.1021/acs.jmedchem.6b01761 BindingDB Entry DOI: 10.7270/Q27P91VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108220 (5-(hydroxymethyl)-2-[3-(4-methoxyphenyl)propyl]- 5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100525 (CHEMBL3327084) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM16452 ((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human Glyoxalase-1 using GSH and MGO as substrate by Dixon plot analysis | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100538 (CHEMBL3327070) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100537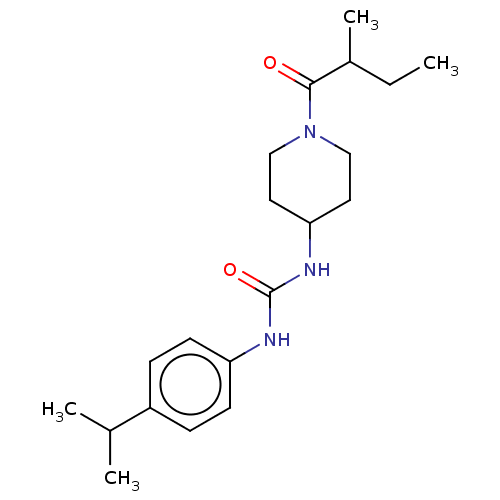 (CHEMBL3327071) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100533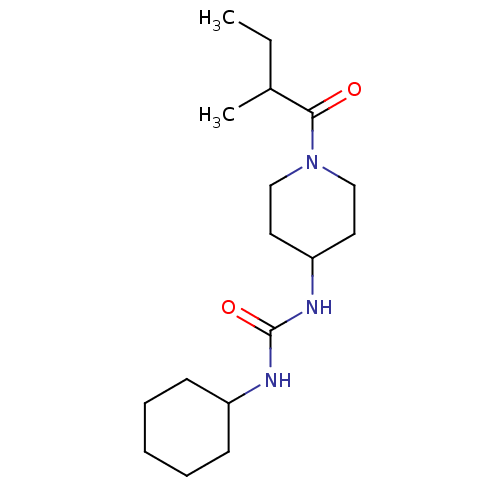 (CHEMBL3327075) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50335967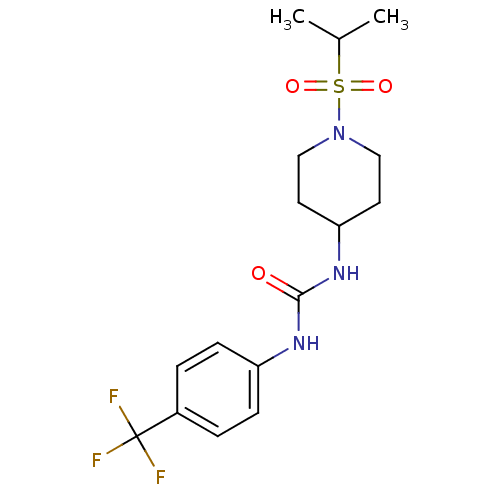 (1-(1-(isopropylsulfonyl)piperidin-4-yl)-3-(4-(trif...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50260065 (CHEMBL4088597 | US9957263, Example 63) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Biology Program, Department of Pharmacology and Toxicology,?Department of Internal Medicine,žSealy Center for Molecular Medicine,?Institute for Translational Sciences, University of Texas Me Curated by ChEMBL | Assay Description Inhibition of His-tagged BRD4 bromodomain 1 (K57 to E168 residues) (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 60: 4533-4558 (2017) Article DOI: 10.1021/acs.jmedchem.6b01761 BindingDB Entry DOI: 10.7270/Q27P91VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50260065 (CHEMBL4088597 | US9957263, Example 63) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Biology Program, Department of Pharmacology and Toxicology,?Department of Internal Medicine,žSealy Center for Molecular Medicine,?Institute for Translational Sciences, University of Texas Me Curated by ChEMBL | Assay Description Inhibition of His-tagged BRD4 bromodomain 1 (K57 to E168 residues) (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 60: 4533-4558 (2017) Article DOI: 10.1021/acs.jmedchem.6b01761 BindingDB Entry DOI: 10.7270/Q27P91VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50143282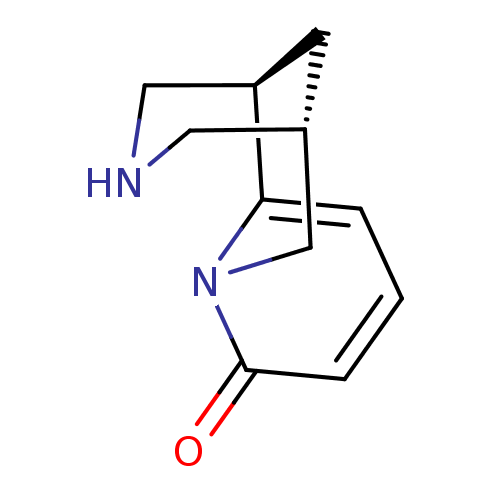 ((-)-cytisine | (1R,5S)-1,2,3,4,5,6-Hexahydro-1,5-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University School of Medicine Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against Nicotinic acetylcholine receptor using [3H]-(-)-nicotine radioligand | Bioorg Med Chem Lett 14: 1845-8 (2004) Article DOI: 10.1016/j.bmcl.2003.09.105 BindingDB Entry DOI: 10.7270/Q2TB17G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50446130 (AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 15942 total ) | Next | Last >> |