| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Leukotriene A-4 hydrolase |
|---|
| Ligand | BDBM50251704 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_488464 (CHEMBL990997) |
|---|
| IC50 | 60±n/a nM |
|---|
| Citation |  Khim, SK; Bauman, J; Evans, J; Freeman, B; King, B; Kirkland, T; Kochanny, M; Lentz, D; Liang, A; Mendoza, L; Phillips, G; Tseng, JL; Wei, RG; Ye, H; Yu, L; Parkinson, J; Guilford, WJ Discovery of novel and potent aryl diamines as leukotriene A4 hydrolase inhibitors. Bioorg Med Chem Lett18:3895-8 (2008) [PubMed] Article Khim, SK; Bauman, J; Evans, J; Freeman, B; King, B; Kirkland, T; Kochanny, M; Lentz, D; Liang, A; Mendoza, L; Phillips, G; Tseng, JL; Wei, RG; Ye, H; Yu, L; Parkinson, J; Guilford, WJ Discovery of novel and potent aryl diamines as leukotriene A4 hydrolase inhibitors. Bioorg Med Chem Lett18:3895-8 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Leukotriene A-4 hydrolase |
|---|
| Name: | Leukotriene A-4 hydrolase |
|---|
| Synonyms: | LKHA4_HUMAN | LTA-4 hydrolase | LTA4 | LTA4H | Leukotriene A(4) hydrolase | Leukotriene A-4 hydrolase (LTA4H) | Leukotriene A4 hydrolase |
|---|
| Type: | Hydrolase; metalloprotease |
|---|
| Mol. Mass.: | 69280.41 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Human recombinant LTA4H. |
|---|
| Residue: | 611 |
|---|
| Sequence: | MPEIVDTCSLASPASVCRTKHLHLRCSVDFTRRTLTGTAALTVQSQEDNLRSLVLDTKDL
TIEKVVINGQEVKYALGERQSYKGSPMEISLPIALSKNQEIVIEISFETSPKSSALQWLT
PEQTSGKEHPYLFSQCQAIHCRAILPCQDTPSVKLTYTAEVSVPKELVALMSAIRDGETP
DPEDPSRKIYKFIQKVPIPCYLIALVVGALESRQIGPRTLVWSEKEQVEKSAYEFSETES
MLKIAEDLGGPYVWGQYDLLVLPPSFPYGGMENPCLTFVTPTLLAGDKSLSNVIAHEISH
SWTGNLVTNKTWDHFWLNEGHTVYLERHICGRLFGEKFRHFNALGGWGELQNSVKTFGET
HPFTKLVVDLTDIDPDVAYSSVPYEKGFALLFYLEQLLGGPEIFLGFLKAYVEKFSYKSI
TTDDWKDFLYSYFKDKVDVLNQVDWNAWLYSPGLPPIKPNYDMTLTNACIALSQRWITAK
EDDLNSFNATDLKDLSSHQLNEFLAQTLQRAPLPLGHIKRMQEVYNFNAINNSEIRFRWL
RLCIQSKWEDAIPLALKMATEQGRMKFTRPLFKDLAAFDKSHDQAVRTYQEHKASMHPVT
AMLVGKDLKVD
|
|
|
|---|
| BDBM50251704 |
|---|
| n/a |
|---|
| Name | BDBM50251704 |
|---|
| Synonyms: | 4-((3-(4-(4-(oxazol-2-yl)phenoxy)phenylamino)piperidin-1-yl)methyl)benzoic acid | CHEMBL479955 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H27N3O4 |
|---|
| Mol. Mass. | 469.5317 |
|---|
| SMILES | OC(=O)c1ccc(CN2CCCC(C2)Nc2ccc(Oc3ccc(cc3)-c3ncco3)cc2)cc1 |
|---|
| Structure | 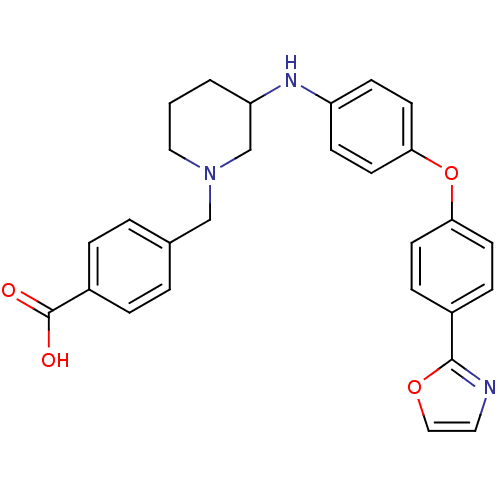
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Khim, SK; Bauman, J; Evans, J; Freeman, B; King, B; Kirkland, T; Kochanny, M; Lentz, D; Liang, A; Mendoza, L; Phillips, G; Tseng, JL; Wei, RG; Ye, H; Yu, L; Parkinson, J; Guilford, WJ Discovery of novel and potent aryl diamines as leukotriene A4 hydrolase inhibitors. Bioorg Med Chem Lett18:3895-8 (2008) [PubMed] Article
Khim, SK; Bauman, J; Evans, J; Freeman, B; King, B; Kirkland, T; Kochanny, M; Lentz, D; Liang, A; Mendoza, L; Phillips, G; Tseng, JL; Wei, RG; Ye, H; Yu, L; Parkinson, J; Guilford, WJ Discovery of novel and potent aryl diamines as leukotriene A4 hydrolase inhibitors. Bioorg Med Chem Lett18:3895-8 (2008) [PubMed] Article