 Found 2207 hits with Last Name = 'evans' and Initial = 'j'
Found 2207 hits with Last Name = 'evans' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5-HT1A receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50395206

(CHEMBL2164354)Show SMILES O=C(C1CCCCC1)N(CCN1CCC(=CC1)c1ccccc1)c1ccccn1 |c:15| Show InChI InChI=1S/C25H31N3O/c29-25(23-11-5-2-6-12-23)28(24-13-7-8-16-26-24)20-19-27-17-14-22(15-18-27)21-9-3-1-4-10-21/h1,3-4,7-10,13-14,16,23H,2,5-6,11-12,15,17-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5-HT1A receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50395206

(CHEMBL2164354)Show SMILES O=C(C1CCCCC1)N(CCN1CCC(=CC1)c1ccccc1)c1ccccn1 |c:15| Show InChI InChI=1S/C25H31N3O/c29-25(23-11-5-2-6-12-23)28(24-13-7-8-16-26-24)20-19-27-17-14-22(15-18-27)21-9-3-1-4-10-21/h1,3-4,7-10,13-14,16,23H,2,5-6,11-12,15,17-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT1A receptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50395207
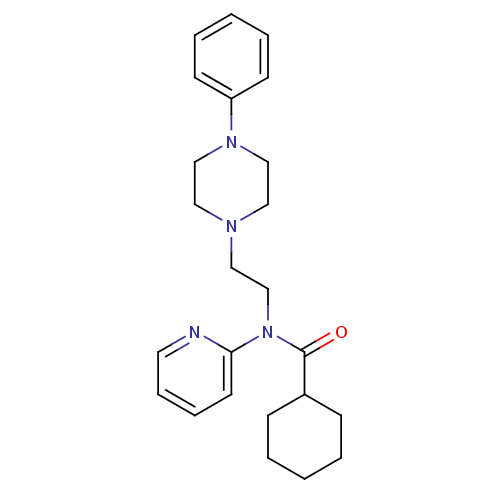
(CHEMBL2164350)Show SMILES O=C(C1CCCCC1)N(CCN1CCN(CC1)c1ccccc1)c1ccccn1 Show InChI InChI=1S/C24H32N4O/c29-24(21-9-3-1-4-10-21)28(23-13-7-8-14-25-23)20-17-26-15-18-27(19-16-26)22-11-5-2-6-12-22/h2,5-8,11-14,21H,1,3-4,9-10,15-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5-HT1A receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50395216

(CHEMBL2164347)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)c2ccc3ccccc3c2)c2ccccn2)CC1 Show InChI InChI=1S/C29H30N4O2/c1-35-27-11-5-4-10-26(27)32-19-16-31(17-20-32)18-21-33(28-12-6-7-15-30-28)29(34)25-14-13-23-8-2-3-9-24(23)22-25/h2-15,22H,16-21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5-HT1A receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50395207

(CHEMBL2164350)Show SMILES O=C(C1CCCCC1)N(CCN1CCN(CC1)c1ccccc1)c1ccccn1 Show InChI InChI=1S/C24H32N4O/c29-24(21-9-3-1-4-10-21)28(23-13-7-8-14-25-23)20-17-26-15-18-27(19-16-26)22-11-5-2-6-12-22/h2,5-8,11-14,21H,1,3-4,9-10,15-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT1A receptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50395215
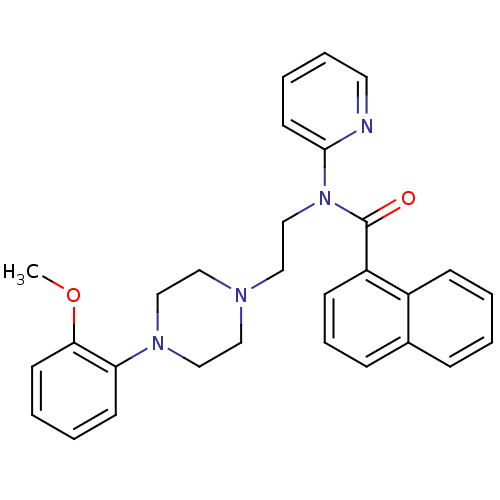
(CHEMBL2164348)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)c2cccc3ccccc23)c2ccccn2)CC1 Show InChI InChI=1S/C29H30N4O2/c1-35-27-14-5-4-13-26(27)32-20-17-31(18-21-32)19-22-33(28-15-6-7-16-30-28)29(34)25-12-8-10-23-9-2-3-11-24(23)25/h2-16H,17-22H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5-HT1A receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50395214

(CHEMBL2164349)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)c2cnc3ccccc3n2)c2ccccn2)CC1 Show InChI InChI=1S/C27H28N6O2/c1-35-25-11-5-4-10-24(25)32-17-14-31(15-18-32)16-19-33(26-12-6-7-13-28-26)27(34)23-20-29-21-8-2-3-9-22(21)30-23/h2-13,20H,14-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5-HT1A receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM81801
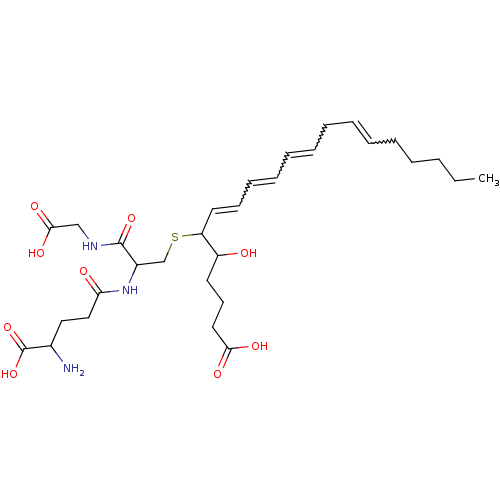
(CAS_5283121 | LTC4 | NSC_5283121)Show SMILES CCCCCC=CCC=CC=CC=CC(SCC(NC(=O)CCC(N)C(O)=O)C(=O)NCC(O)=O)C(O)CCCC(O)=O |w:5.4,8.7,10.9,12.11| Show InChI InChI=1S/C30H47N3O9S/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-25(24(34)15-14-17-27(36)37)43-21-23(29(40)32-20-28(38)39)33-26(35)19-18-22(31)30(41)42/h6-7,9-13,16,22-25,34H,2-5,8,14-15,17-21,31H2,1H3,(H,32,40)(H,33,35)(H,36,37)(H,38,39)(H,41,42) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by PDSP Ki Database
| |
J Biol Chem 275: 30531-6 (2000)
Article DOI: 10.1074/jbc.M003490200
BindingDB Entry DOI: 10.7270/Q2BP01BH |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM50292408
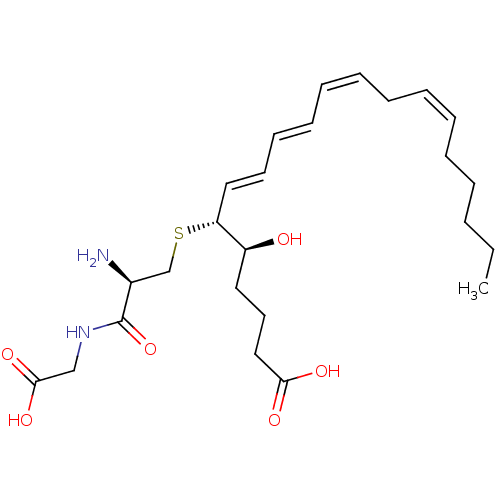
((R-(R*,S*-(E,E,Z,Z)))-N-(S-(1-(4-Carboxy-1-hydroxy...)Show SMILES CCCCC\C=C/C\C=C/C=C/C=C/[C@@H](SC[C@H](N)C(=O)NCC(O)=O)[C@@H](O)CCCC(O)=O |r| Show InChI InChI=1S/C25H40N2O6S/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-22(21(28)15-14-17-23(29)30)34-19-20(26)25(33)27-18-24(31)32/h6-7,9-13,16,20-22,28H,2-5,8,14-15,17-19,26H2,1H3,(H,27,33)(H,29,30)(H,31,32)/b7-6-,10-9-,12-11+,16-13+/t20-,21-,22+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by PDSP Ki Database
| |
J Biol Chem 275: 30531-6 (2000)
Article DOI: 10.1074/jbc.M003490200
BindingDB Entry DOI: 10.7270/Q2BP01BH |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50395208

(CHEMBL2164346)Show SMILES O=C(N(CCN1CCC(=CC1)c1ccccc1)c1ccccn1)c1cnc2ccccc2n1 |c:8| Show InChI InChI=1S/C27H25N5O/c33-27(25-20-29-23-10-4-5-11-24(23)30-25)32(26-12-6-7-15-28-26)19-18-31-16-13-22(14-17-31)21-8-2-1-3-9-21/h1-13,15,20H,14,16-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5-HT1A receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50395210

(CHEMBL2164355)Show SMILES O=C(N(CCN1CCC(=CC1)c1ccccc1)c1ccccn1)c1ccc2ccccc2c1 |c:8| Show InChI InChI=1S/C29H27N3O/c33-29(27-14-13-24-10-4-5-11-26(24)22-27)32(28-12-6-7-17-30-28)21-20-31-18-15-25(16-19-31)23-8-2-1-3-9-23/h1-15,17,22H,16,18-21H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5-HT1A receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(RAT) | BDBM50217230
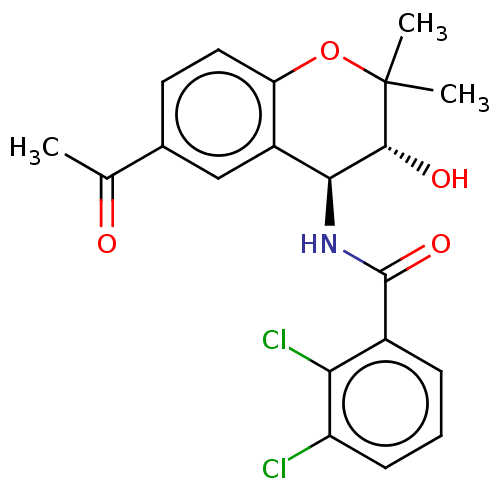
(CHEMBL95387)Show SMILES CC(=O)c1ccc2OC(C)(C)[C@H](O)[C@@H](NC(=O)c3cccc(Cl)c3Cl)c2c1 Show InChI InChI=1S/C20H19Cl2NO4/c1-10(24)11-7-8-15-13(9-11)17(18(25)20(2,3)27-15)23-19(26)12-5-4-6-14(21)16(12)22/h4-9,17-18,25H,1-3H3,(H,23,26)/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 1D receptor in the rat forebrain by [3H]- SB-204269 displacement. |
Bioorg Med Chem Lett 9: 285-90 (1999)
BindingDB Entry DOI: 10.7270/Q2VD71N3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(RAT) | BDBM50217225
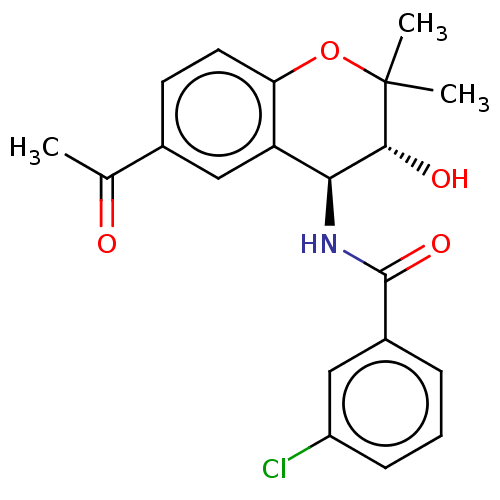
(CHEMBL98434)Show SMILES CC(=O)c1ccc2OC(C)(C)[C@H](O)[C@@H](NC(=O)c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C20H20ClNO4/c1-11(23)12-7-8-16-15(10-12)17(18(24)20(2,3)26-16)22-19(25)13-5-4-6-14(21)9-13/h4-10,17-18,24H,1-3H3,(H,22,25)/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 1D receptor in the rat forebrain by [3H]- SB-204269 displacement. |
Bioorg Med Chem Lett 9: 285-90 (1999)
BindingDB Entry DOI: 10.7270/Q2VD71N3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(RAT) | BDBM50217226

(CHEMBL318906)Show SMILES CC(=O)c1ccc2OC(C)(C)[C@@H](O)[C@@H](NC(=O)c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C20H20ClNO4/c1-11(23)12-7-8-16-15(10-12)17(18(24)20(2,3)26-16)22-19(25)13-5-4-6-14(21)9-13/h4-10,17-18,24H,1-3H3,(H,22,25)/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 1D receptor in the rat forebrain by [3H]- SB-204269 displacement. |
Bioorg Med Chem Lett 9: 285-90 (1999)
BindingDB Entry DOI: 10.7270/Q2VD71N3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(RAT) | BDBM50217210
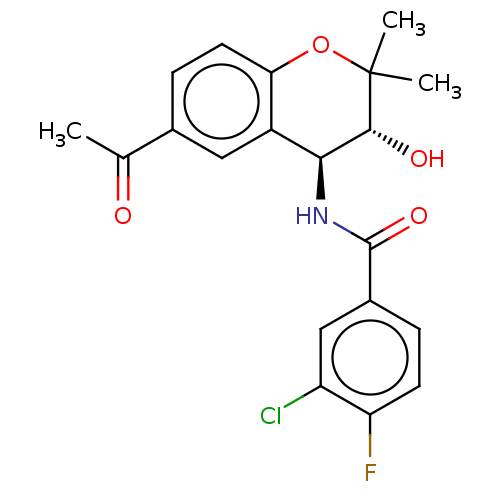
(CHEMBL38918)Show SMILES CC(=O)c1ccc2OC(C)(C)[C@H](O)[C@@H](NC(=O)c3ccc(F)c(Cl)c3)c2c1 Show InChI InChI=1S/C20H19ClFNO4/c1-10(24)11-5-7-16-13(8-11)17(18(25)20(2,3)27-16)23-19(26)12-4-6-15(22)14(21)9-12/h4-9,17-18,25H,1-3H3,(H,23,26)/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 1D receptor in the rat forebrain by [3H]- SB-204269 displacement. |
Bioorg Med Chem Lett 9: 285-90 (1999)
BindingDB Entry DOI: 10.7270/Q2VD71N3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50395209

(CHEMBL2164345)Show SMILES O=C(N(CCN1CCC(=CC1)c1ccccc1)c1ccccn1)c1cccc2ccccc12 |c:8| Show InChI InChI=1S/C29H27N3O/c33-29(27-14-8-12-25-11-4-5-13-26(25)27)32(28-15-6-7-18-30-28)22-21-31-19-16-24(17-20-31)23-9-2-1-3-10-23/h1-16,18H,17,19-22H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5-HT1A receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50395214

(CHEMBL2164349)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)c2cnc3ccccc3n2)c2ccccn2)CC1 Show InChI InChI=1S/C27H28N6O2/c1-35-25-11-5-4-10-24(25)32-17-14-31(15-18-32)16-19-33(26-12-6-7-13-28-26)27(34)23-20-29-21-8-2-3-9-22(21)30-23/h2-13,20H,14-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM-09151-2 from human cloned D4 receptor expressed in Sf9 cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(RAT) | BDBM50217211
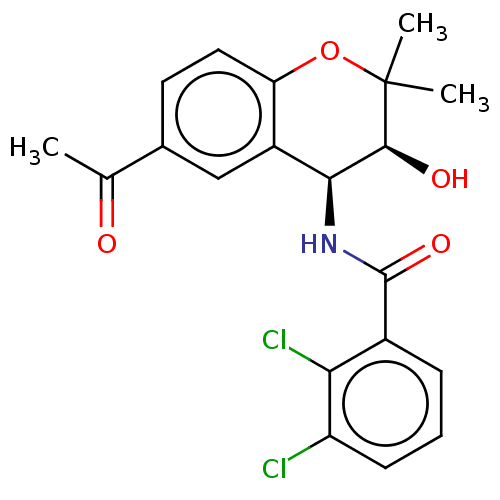
(CHEMBL318022)Show SMILES CC(=O)c1ccc2OC(C)(C)[C@@H](O)[C@@H](NC(=O)c3cccc(Cl)c3Cl)c2c1 Show InChI InChI=1S/C20H19Cl2NO4/c1-10(24)11-7-8-15-13(9-11)17(18(25)20(2,3)27-15)23-19(26)12-5-4-6-14(21)16(12)22/h4-9,17-18,25H,1-3H3,(H,23,26)/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 1D receptor in the rat forebrain by [3H]- SB-204269 displacement. |
Bioorg Med Chem Lett 9: 285-90 (1999)
BindingDB Entry DOI: 10.7270/Q2VD71N3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(RAT) | BDBM50217182

(CHEMBL318882)Show SMILES CC(=O)c1ccc2OC(C)(C)[C@H](O)[C@@H](NC(=O)c3ccsc3Cl)c2c1 Show InChI InChI=1S/C18H18ClNO4S/c1-9(21)10-4-5-13-12(8-10)14(15(22)18(2,3)24-13)20-17(23)11-6-7-25-16(11)19/h4-8,14-15,22H,1-3H3,(H,20,23)/t14-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 1D receptor in the rat forebrain by [3H]- SB-204269 displacement. |
Bioorg Med Chem Lett 9: 285-90 (1999)
BindingDB Entry DOI: 10.7270/Q2VD71N3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50395213

(CHEMBL2164351)Show SMILES O=C(N(CCN1CCN(CC1)c1ccccc1)c1ccccn1)c1ccc2ccccc2c1 Show InChI InChI=1S/C28H28N4O/c33-28(25-14-13-23-8-4-5-9-24(23)22-25)32(27-12-6-7-15-29-27)21-18-30-16-19-31(20-17-30)26-10-2-1-3-11-26/h1-15,22H,16-21H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5-HT1A receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 19.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(RAT) | BDBM50217231
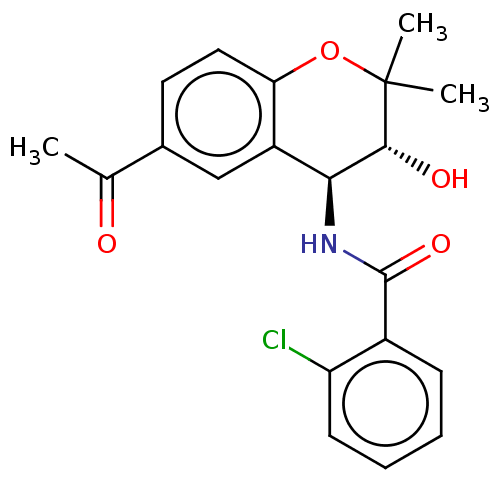
(CHEMBL98433)Show SMILES CC(=O)c1ccc2OC(C)(C)[C@H](O)[C@@H](NC(=O)c3ccccc3Cl)c2c1 Show InChI InChI=1S/C20H20ClNO4/c1-11(23)12-8-9-16-14(10-12)17(18(24)20(2,3)26-16)22-19(25)13-6-4-5-7-15(13)21/h4-10,17-18,24H,1-3H3,(H,22,25)/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 1D receptor in the rat forebrain by [3H]- SB-204269 displacement. |
Bioorg Med Chem Lett 9: 285-90 (1999)
BindingDB Entry DOI: 10.7270/Q2VD71N3 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(RAT) | BDBM50217229

(CHEMBL317732)Show SMILES CC(=O)c1ccc2OC(C)(C)[C@H](O)[C@@H](NC(=O)c3ccc(F)cc3Cl)c2c1 Show InChI InChI=1S/C20H19ClFNO4/c1-10(24)11-4-7-16-14(8-11)17(18(25)20(2,3)27-16)23-19(26)13-6-5-12(22)9-15(13)21/h4-9,17-18,25H,1-3H3,(H,23,26)/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 1D receptor in the rat forebrain by [3H]- SB-204269 displacement. |
Bioorg Med Chem Lett 9: 285-90 (1999)
BindingDB Entry DOI: 10.7270/Q2VD71N3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(RAT) | BDBM50217183

(CHEMBL316885)Show SMILES CC(=O)c1ccc2OC(C)(C)[C@@H](O)[C@@H](NC(=O)c3ccccc3Cl)c2c1 Show InChI InChI=1S/C20H20ClNO4/c1-11(23)12-8-9-16-14(10-12)17(18(24)20(2,3)26-16)22-19(25)13-6-4-5-7-15(13)21/h4-10,17-18,24H,1-3H3,(H,22,25)/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 1D receptor in the rat forebrain by [3H]- SB-204269 displacement. |
Bioorg Med Chem Lett 9: 285-90 (1999)
BindingDB Entry DOI: 10.7270/Q2VD71N3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(RAT) | BDBM50217178

(CHEMBL99341)Show SMILES CC(=O)c1ccc2OC(C)(C)[C@H](O)[C@@H](NC(=O)c3cc(Cl)cc(Cl)c3Cl)c2c1 Show InChI InChI=1S/C20H18Cl3NO4/c1-9(25)10-4-5-15-12(6-10)17(18(26)20(2,3)28-15)24-19(27)13-7-11(21)8-14(22)16(13)23/h4-8,17-18,26H,1-3H3,(H,24,27)/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 1D receptor in the rat forebrain by [3H]- SB-204269 displacement. |
Bioorg Med Chem Lett 9: 285-90 (1999)
BindingDB Entry DOI: 10.7270/Q2VD71N3 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50333880
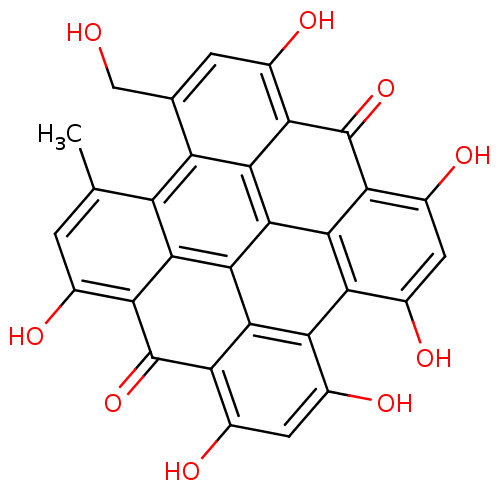
(CHEMBL1614664 | Hypericin | Pseudohypericin)Show SMILES Cc1cc(O)c2c3c1c1c(CO)cc(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O Show InChI InChI=1S/C30H16O9/c1-7-2-9(32)19-23-15(7)16-8(6-31)3-10(33)20-24(16)28-26-18(12(35)5-14(37)22(26)30(20)39)17-11(34)4-13(36)21(29(19)38)25(17)27(23)28/h2-5,31-37H,6H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 34.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50395208

(CHEMBL2164346)Show SMILES O=C(N(CCN1CCC(=CC1)c1ccccc1)c1ccccn1)c1cnc2ccccc2n1 |c:8| Show InChI InChI=1S/C27H25N5O/c33-27(25-20-29-23-10-4-5-11-24(23)30-25)32(26-12-6-7-15-28-26)19-18-31-16-13-22(14-17-31)21-8-2-1-3-9-21/h1-13,15,20H,14,16-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM-09151-2 from human cloned D4 receptor expressed in Sf9 cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50217942
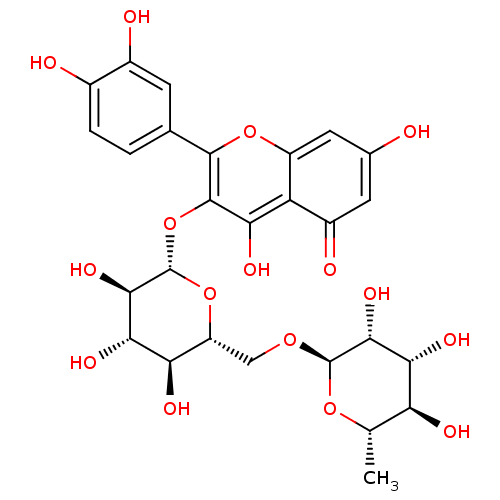
(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chr...)Show SMILES C[C@@H]1O[C@@H](OC[C@H]2O[C@@H](Oc3c(O)c4c(cc(O)cc4=O)oc3-c3ccc(O)c(O)c3)[C@H](O)[C@@H](O)[C@@H]2O)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C27H30O16/c1-8-17(32)20(35)22(37)26(40-8)39-7-15-18(33)21(36)23(38)27(42-15)43-25-19(34)16-13(31)5-10(28)6-14(16)41-24(25)9-2-3-11(29)12(30)4-9/h2-6,8,15,17-18,20-23,26-30,32-38H,7H2,1H3/t8-,15+,17-,18+,20+,21-,22+,23+,26+,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 35.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50395212

(CHEMBL2164352)Show SMILES O=C(N(CCN1CCN(CC1)c1ccccc1)c1ccccn1)c1cccc2ccccc12 Show InChI InChI=1S/C28H28N4O/c33-28(26-14-8-10-23-9-4-5-13-25(23)26)32(27-15-6-7-16-29-27)22-19-30-17-20-31(21-18-30)24-11-2-1-3-12-24/h1-16H,17-22H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5-HT1A receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50395211
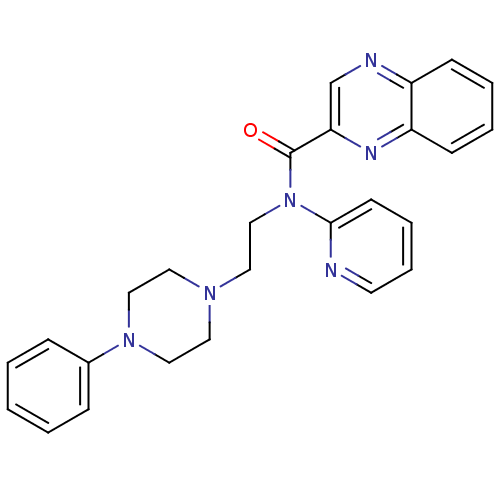
(CHEMBL2164353)Show SMILES O=C(N(CCN1CCN(CC1)c1ccccc1)c1ccccn1)c1cnc2ccccc2n1 Show InChI InChI=1S/C26H26N6O/c33-26(24-20-28-22-10-4-5-11-23(22)29-24)32(25-12-6-7-13-27-25)19-16-30-14-17-31(18-15-30)21-8-2-1-3-9-21/h1-13,20H,14-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5-HT1A receptor expressed in CHO cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 36.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50395206

(CHEMBL2164354)Show SMILES O=C(C1CCCCC1)N(CCN1CCC(=CC1)c1ccccc1)c1ccccn1 |c:15| Show InChI InChI=1S/C25H31N3O/c29-25(23-11-5-2-6-12-23)28(24-13-7-8-16-26-24)20-19-27-17-14-22(15-18-27)21-9-3-1-4-10-21/h1,3-4,7-10,13-14,16,23H,2,5-6,11-12,15,17-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Binding affinity to adrenergic alpha1A receptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50174201
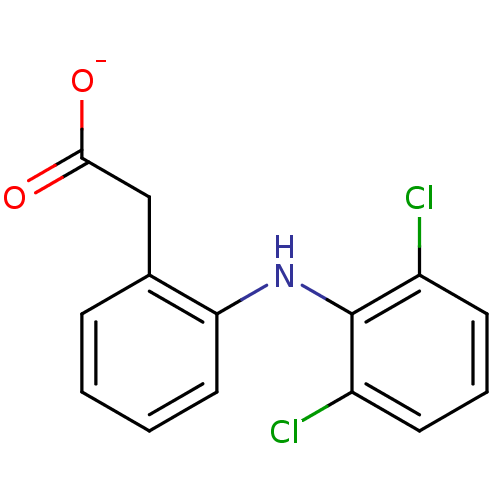
(ARTHROTEC | GP 45840 | SOLARAZE | Sodium; [2-(2,6-...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM-09151-2 from human cloned D4 receptor expressed in Sf9 cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50395206

(CHEMBL2164354)Show SMILES O=C(C1CCCCC1)N(CCN1CCC(=CC1)c1ccccc1)c1ccccn1 |c:15| Show InChI InChI=1S/C25H31N3O/c29-25(23-11-5-2-6-12-23)28(24-13-7-8-16-26-24)20-19-27-17-14-22(15-18-27)21-9-3-1-4-10-21/h1,3-4,7-10,13-14,16,23H,2,5-6,11-12,15,17-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT2A receptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50395206

(CHEMBL2164354)Show SMILES O=C(C1CCCCC1)N(CCN1CCC(=CC1)c1ccccc1)c1ccccn1 |c:15| Show InChI InChI=1S/C25H31N3O/c29-25(23-11-5-2-6-12-23)28(24-13-7-8-16-26-24)20-19-27-17-14-22(15-18-27)21-9-3-1-4-10-21/h1,3-4,7-10,13-14,16,23H,2,5-6,11-12,15,17-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D4 receptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50395211

(CHEMBL2164353)Show SMILES O=C(N(CCN1CCN(CC1)c1ccccc1)c1ccccn1)c1cnc2ccccc2n1 Show InChI InChI=1S/C26H26N6O/c33-26(24-20-28-22-10-4-5-11-23(22)29-24)32(25-12-6-7-13-27-25)19-16-30-14-17-31(18-15-30)21-8-2-1-3-9-21/h1-13,20H,14-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Li£ge
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM-09151-2 from human cloned D4 receptor expressed in Sf9 cells after 60 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 4550-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.119
BindingDB Entry DOI: 10.7270/Q2HH6M6V |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data