| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Type-1 angiotensin II receptor B |
|---|
| Ligand | BDBM50282321 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_34944 (CHEMBL647787) |
|---|
| Ki | 32±n/a nM |
|---|
| Citation |  Lloyd, J; Ryono, DE; Bird, JE; Buote, J; Delaney, CL; Dejneka, T; Dickinson, KE; Moreland, S; Normandin, DE; Skwish, S; Spitzmiller, ER; Waldron, TL Quinoline-4-carboxylic acids as angiotensin II receptor antagonists Bioorg Med Chem Lett4:195-200 (1994) Article Lloyd, J; Ryono, DE; Bird, JE; Buote, J; Delaney, CL; Dejneka, T; Dickinson, KE; Moreland, S; Normandin, DE; Skwish, S; Spitzmiller, ER; Waldron, TL Quinoline-4-carboxylic acids as angiotensin II receptor antagonists Bioorg Med Chem Lett4:195-200 (1994) Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Type-1 angiotensin II receptor B |
|---|
| Name: | Type-1 angiotensin II receptor B |
|---|
| Synonyms: | AGTRB_RAT | AT3 | Agtr1 | Agtr1b | Angiotensin II AT1B | Angiotensin II receptor (AT-1) type-1 | Angiotensin II type 1b (AT-1b) receptor | At1b | Type-1B angiotensin II receptor |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 40929.44 |
|---|
| Organism: | RAT |
|---|
| Description: | Angiotensin II AT1B 0 RAT::P29089 |
|---|
| Residue: | 359 |
|---|
| Sequence: | MTLNSSTEDGIKRIQDDCPKAGRHNYIFVMIPTLYSIIFVVGIFGNSLVVIVIYFYMKLK
TVASVFLLNLALADLCFLLTLPLWAVYTAMEYRWPFGNHLCKIASASVSFNLYASVFLLT
CLSIDRYLAIVHPMKSRLRRTMLVAKVTCIIIWLMAGLASLPAVIYRNVYFIENTNITVC
AFHYESQNSTLPIGLGLTKNILGFVFPFLIILTSYTLIWKALKKAYKIQKNTPRNDDIFR
IIMAIVLFFFFSWVPHQIFTFLDVLIQLGIIRDCEIADIVDTAMPITICIAYFNNCLNPL
FYGFLGKKFKKYFLQLLKYIPPTAKSHAGLSTKMSTLSYRPSDNMSSSAKKSASFFEVE
|
|
|
|---|
| BDBM50282321 |
|---|
| n/a |
|---|
| Name | BDBM50282321 |
|---|
| Synonyms: | 7-Chloro-2-cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-yloxy]-quinoline-4-carboxylic acid | CHEMBL64567 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H18ClN5O3 |
|---|
| Mol. Mass. | 483.906 |
|---|
| SMILES | OC(=O)c1c(Oc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(nc2cc(Cl)ccc12)C1CC1 |
|---|
| Structure | 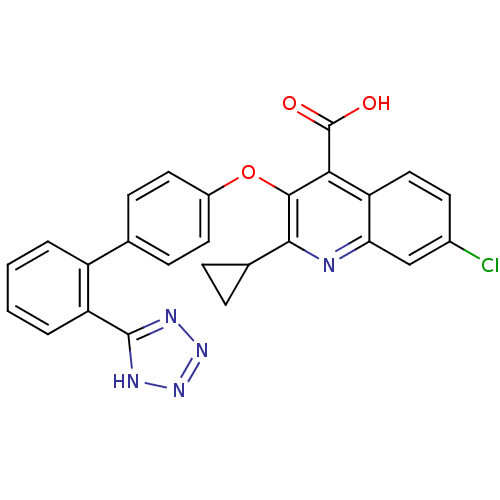
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Lloyd, J; Ryono, DE; Bird, JE; Buote, J; Delaney, CL; Dejneka, T; Dickinson, KE; Moreland, S; Normandin, DE; Skwish, S; Spitzmiller, ER; Waldron, TL Quinoline-4-carboxylic acids as angiotensin II receptor antagonists Bioorg Med Chem Lett4:195-200 (1994) Article
Lloyd, J; Ryono, DE; Bird, JE; Buote, J; Delaney, CL; Dejneka, T; Dickinson, KE; Moreland, S; Normandin, DE; Skwish, S; Spitzmiller, ER; Waldron, TL Quinoline-4-carboxylic acids as angiotensin II receptor antagonists Bioorg Med Chem Lett4:195-200 (1994) Article