

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231010 (CHEMBL312066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449917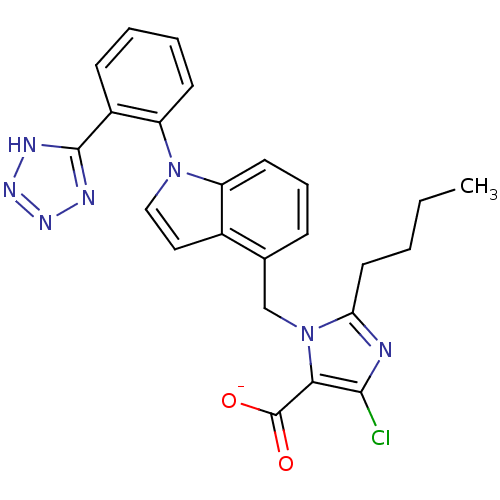 (BMS-180560 | CHEMBL2021417) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231010 (CHEMBL312066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231010 (CHEMBL312066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.01% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50106836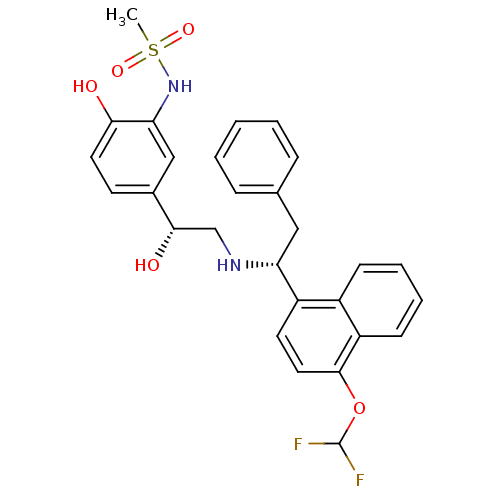 (CHEMBL105758 | N-(5-{(R)-2-[(R)-1-(4-Difluorometho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to CHO cells expressing the cloned human beta-3 adrenergic receptor in the presence of [125I]-iodocyanopindolol | Bioorg Med Chem Lett 11: 3041-4 (2001) BindingDB Entry DOI: 10.7270/Q2794402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282324 (2-Cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50049201 (2-Cyclopropyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214244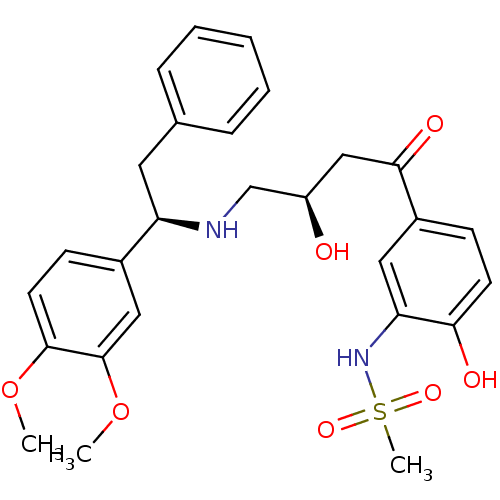 (CHEMBL250978 | N-(5-((R)-4-((R)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282318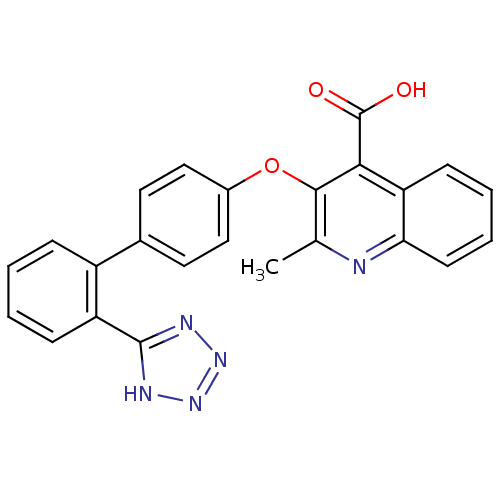 (2-Methyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-yloxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282322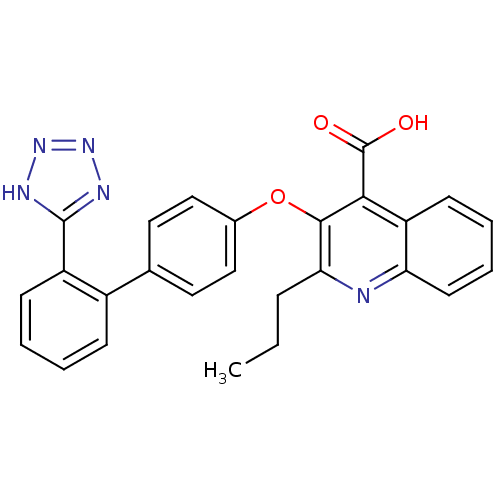 (2-Propyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-yloxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449914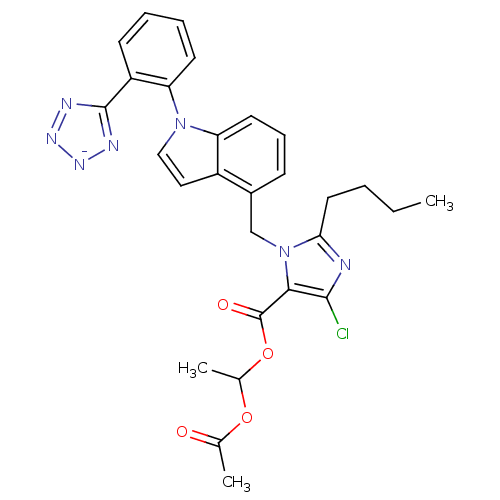 (CHEMBL2079784) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231140 (CHEMBL77029) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282316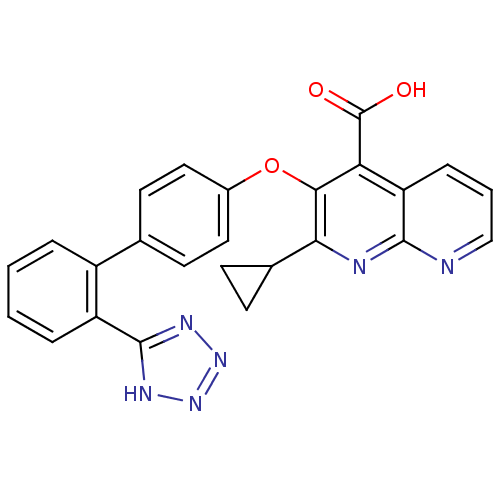 (2-Cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214246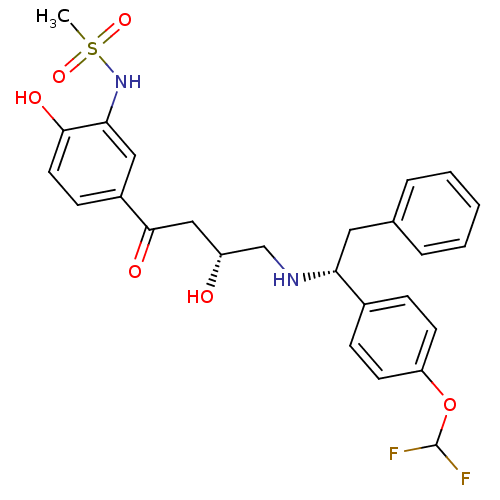 (CHEMBL401135 | N-(5-((R)-4-((R)-1-(4-(difluorometh...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231145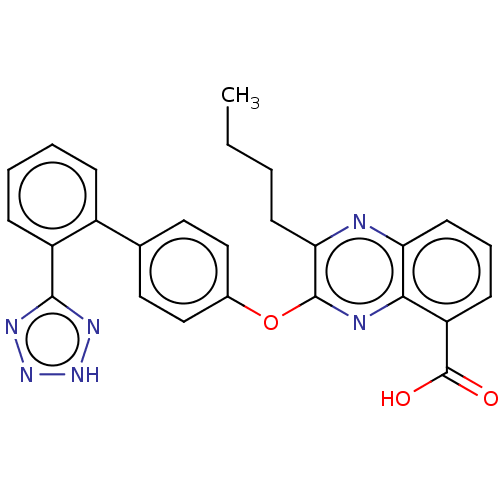 (CHEMBL76785) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0% against rat Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282315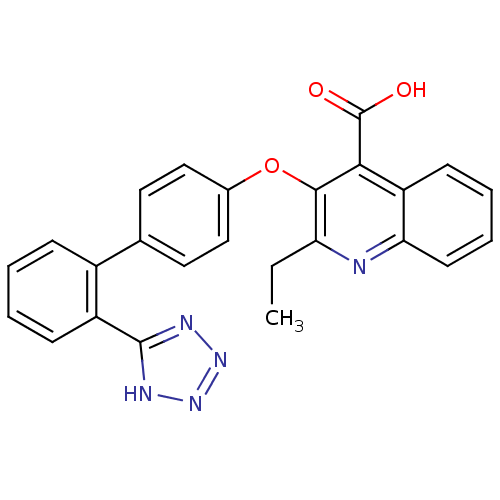 (2-Ethyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-yloxy]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214234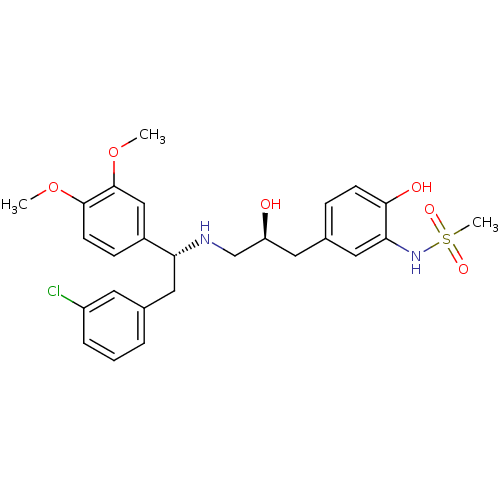 (CHEMBL250755 | N-(5-((S)-3-((R)-2-(3-chlorophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214213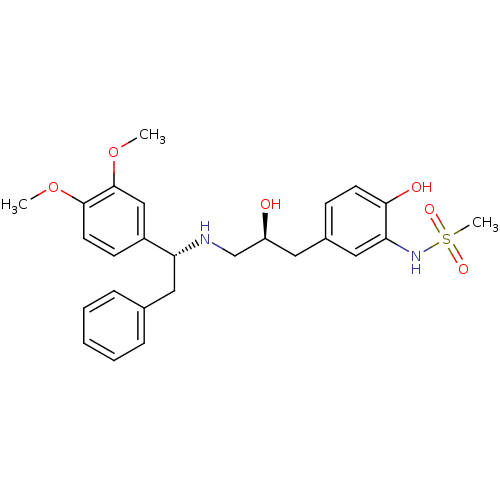 (CHEMBL399329 | N-(5-((S)-3-((R)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50366331 (CHEMBL1790055) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449919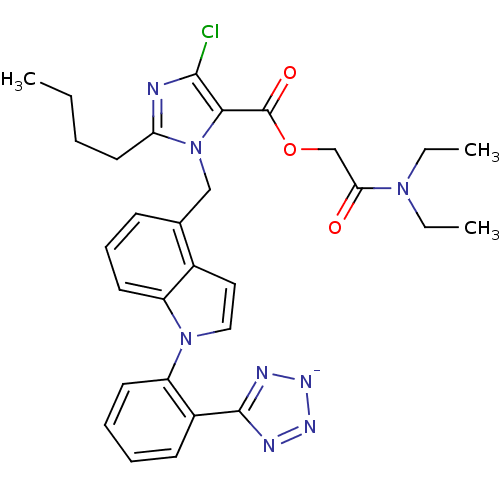 (CHEMBL2021415) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214240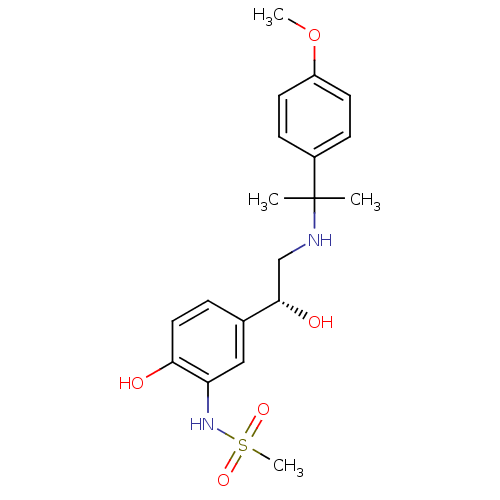 ((R)-N-(2-hydroxy-5-(1-hydroxy-2-(2-(4-methoxypheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214242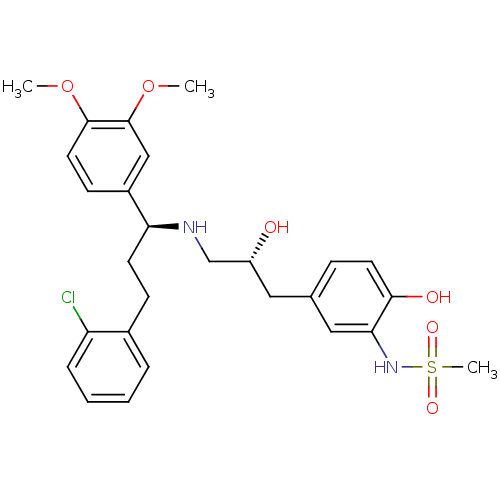 (CHEMBL447786 | N-(5-((R)-3-((S)-3-(2-chlorophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214248 (CHEMBL437578 | N-(5-((S)-3-((R)-2-(2-chlorophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449910 (CHEMBL2079782) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231150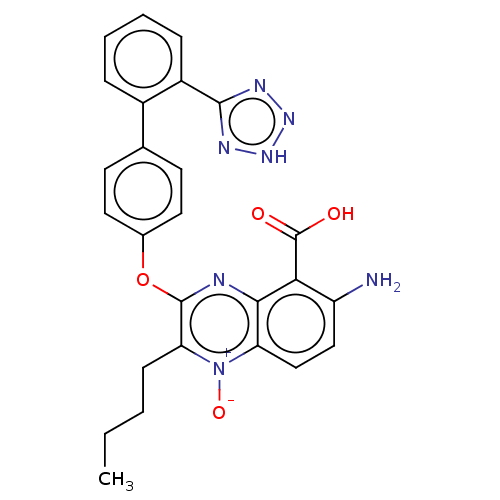 (CHEMBL77935) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50106852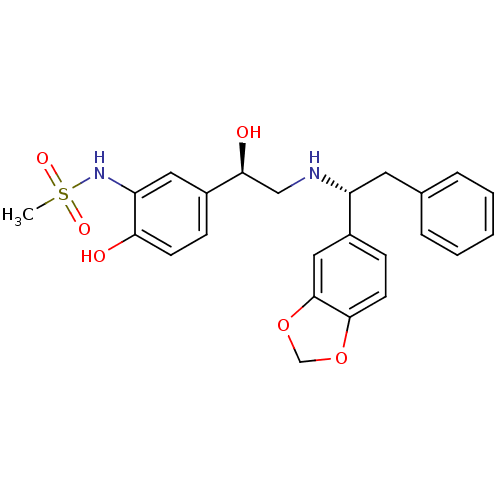 (CHEMBL321518 | N-{5-[(R)-2-((R)-1-Benzo[1,3]dioxol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to CHO cells expressing the cloned human beta-3 adrenergic receptor in the presence of [125I]-iodocyanopindolol | Bioorg Med Chem Lett 11: 3041-4 (2001) BindingDB Entry DOI: 10.7270/Q2794402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214237 (CHEMBL400947 | N-(2-hydroxy-5-((S)-2-hydroxy-3-((R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50148497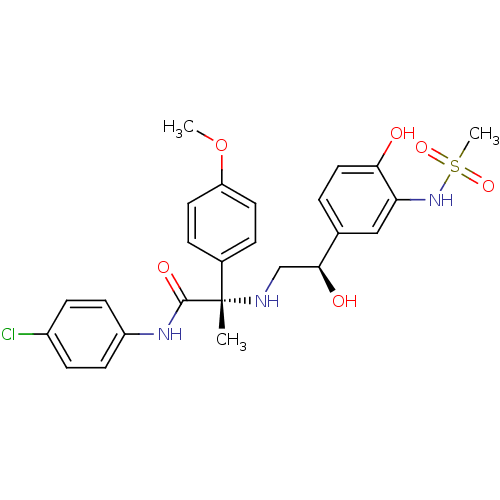 ((S)-N-(4-Chloro-phenyl)-2-[2-hydroxy-2-((R)-4-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human beta-3 adrenergic receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 3525-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.074 BindingDB Entry DOI: 10.7270/Q2MP52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449912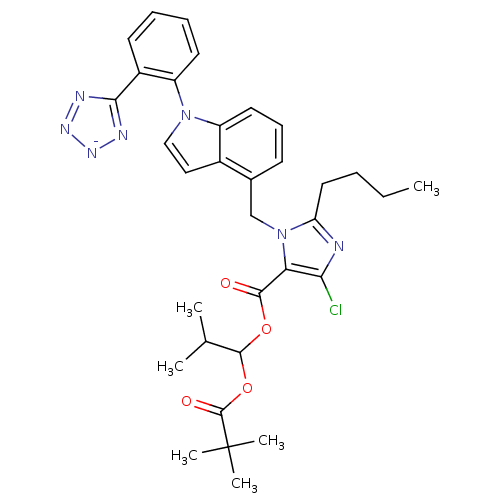 (CHEMBL2079781) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231139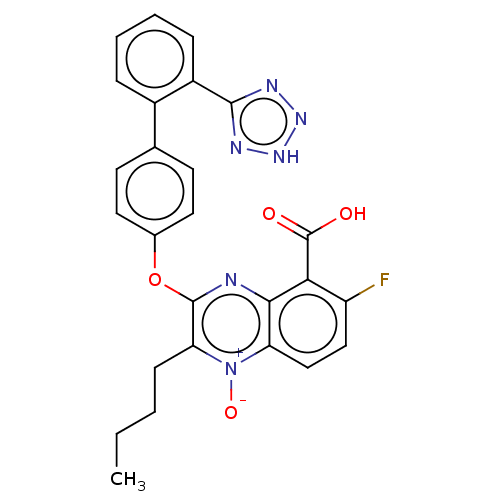 (CHEMBL77827) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214228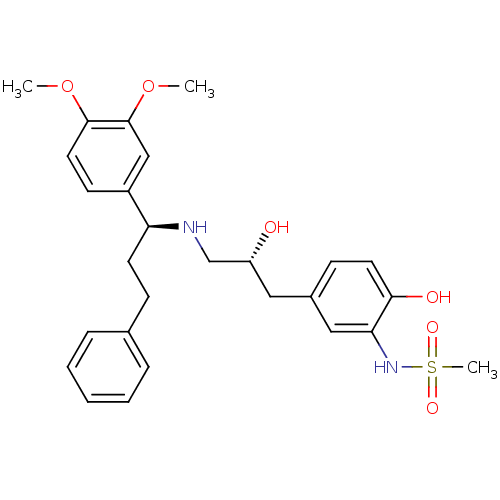 (CHEMBL400067 | N-(5-((R)-3-((S)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214225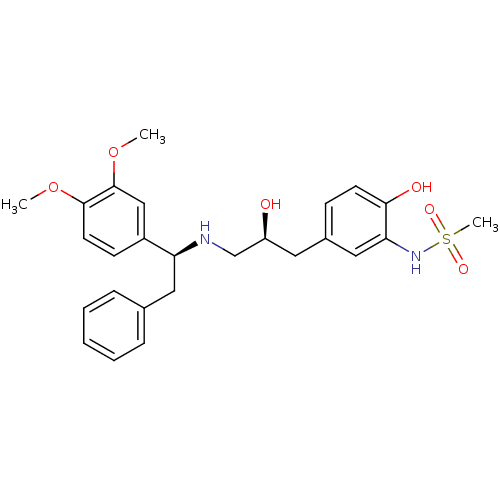 (CHEMBL398557 | N-(5-((S)-3-((S)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50106833 (CHEMBL317648 | N-(5-{(R)-2-[(R)-1-(4-Difluorometho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to CHO cells expressing the cloned human beta-3 adrenergic receptor in the presence of [125I]-iodocyanopindolol | Bioorg Med Chem Lett 11: 3041-4 (2001) BindingDB Entry DOI: 10.7270/Q2794402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50106810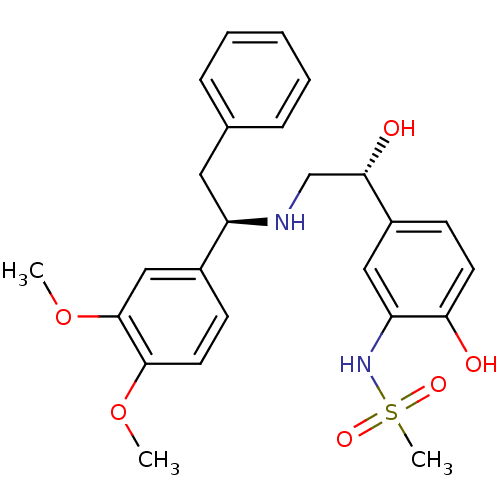 (CHEMBL317621 | N-(5-((R)-2-((R)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against CHO cells transfected with human beta-3 adrenergic receptor in the presence of [125I]iodocyanopindolol | Bioorg Med Chem Lett 11: 3035-9 (2001) BindingDB Entry DOI: 10.7270/Q2MS3T97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50106810 (CHEMBL317621 | N-(5-((R)-2-((R)-1-(3,4-dimethoxyph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50148487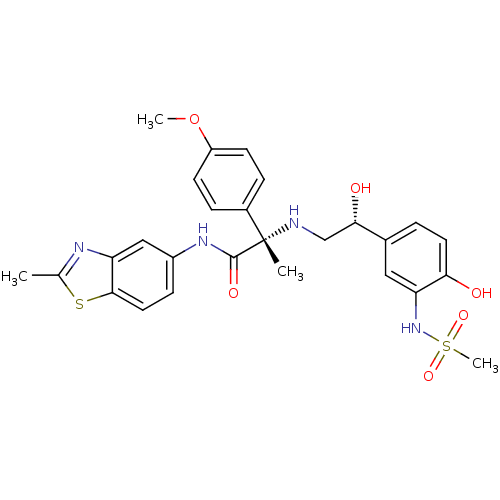 ((S)-2-[2-Hydroxy-2-((R)-4-hydroxy-3-methanesulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human beta-3 adrenergic receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 3525-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.074 BindingDB Entry DOI: 10.7270/Q2MP52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214227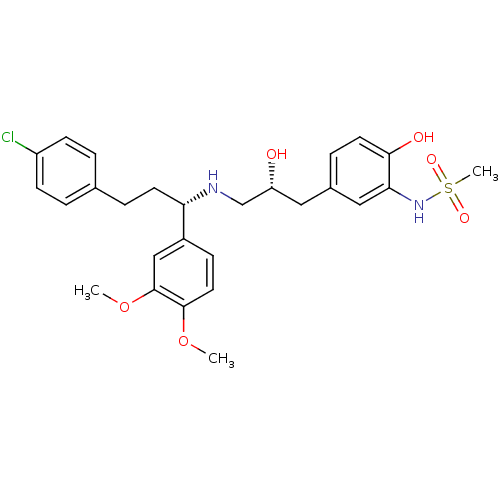 (CHEMBL251764 | N-(5-((R)-3-((S)-3-(4-chlorophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282317 (2-Cyclopropyl-5-methyl-3-[2'-(2H-tetrazol-5-yl)-bi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449909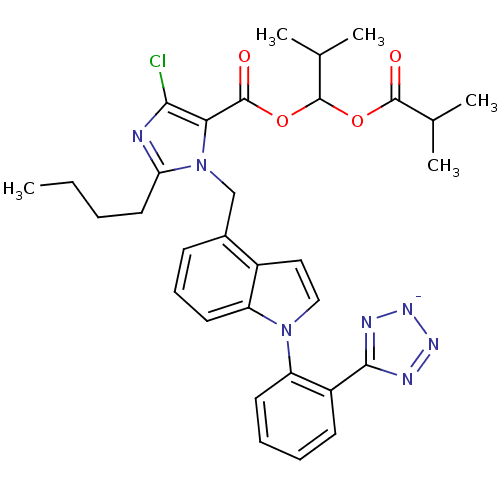 (CHEMBL2079769) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50148484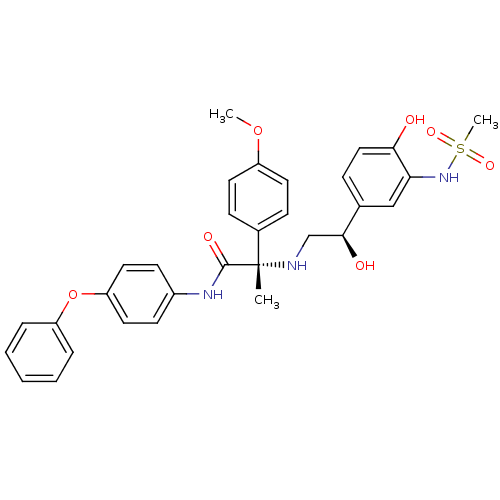 ((S)-2-[2-Hydroxy-2-((R)-4-hydroxy-3-methanesulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human beta-3 adrenergic receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 3525-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.074 BindingDB Entry DOI: 10.7270/Q2MP52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282319 (2-Cyclopropyl-6-fluoro-3-[2'-(2H-tetrazol-5-yl)-bi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50106825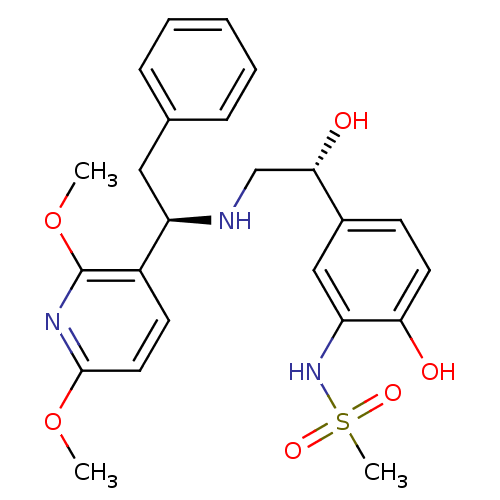 (CHEMBL102611 | N-(5-{(R)-2-[(R)-1-(2,6-Dimethoxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to CHO cells expressing the cloned human beta-3 adrenergic receptor in the presence of [125I]-iodocyanopindolol | Bioorg Med Chem Lett 11: 3041-4 (2001) BindingDB Entry DOI: 10.7270/Q2794402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231149 (CHEMBL306278) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231143 (CHEMBL75053) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50148507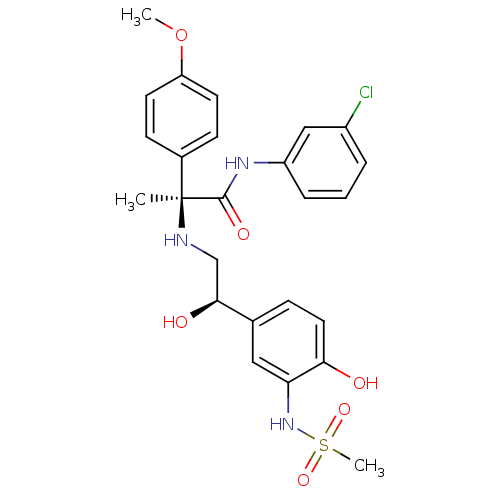 ((S)-N-(3-Chloro-phenyl)-2-[2-hydroxy-2-((R)-4-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human beta-3 adrenergic receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 3525-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.074 BindingDB Entry DOI: 10.7270/Q2MP52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50148486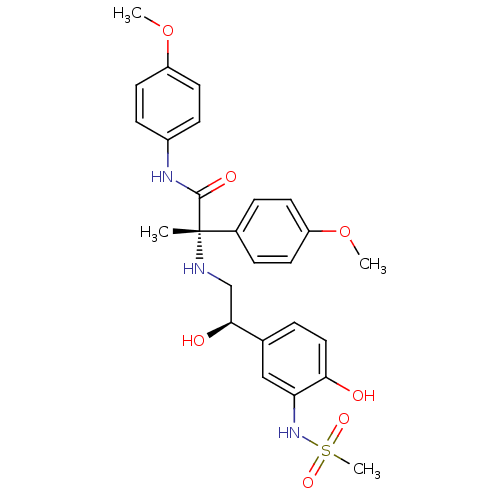 ((S)-2-[2-Hydroxy-2-((R)-4-hydroxy-3-methanesulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human beta-3 adrenergic receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 3525-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.074 BindingDB Entry DOI: 10.7270/Q2MP52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50214233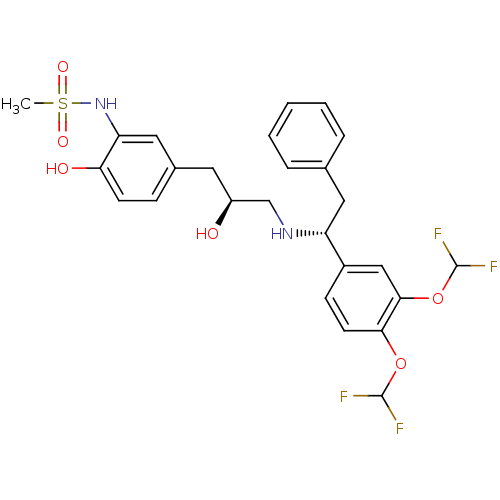 (CHEMBL250553 | N-(5-((S)-3-((R)-1-(3,4-bis(difluor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from cloned human adrenergic beta-3 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 4290-6 (2007) Article DOI: 10.1016/j.bmcl.2007.05.030 BindingDB Entry DOI: 10.7270/Q2M61JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50148520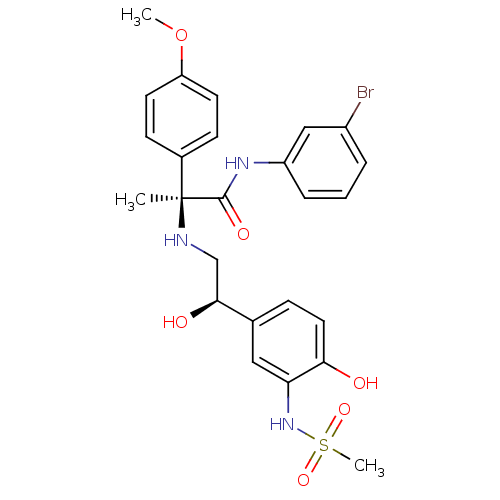 ((S)-N-(3-Bromo-phenyl)-2-[2-hydroxy-2-((R)-4-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human beta-3 adrenergic receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 3525-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.074 BindingDB Entry DOI: 10.7270/Q2MP52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50148504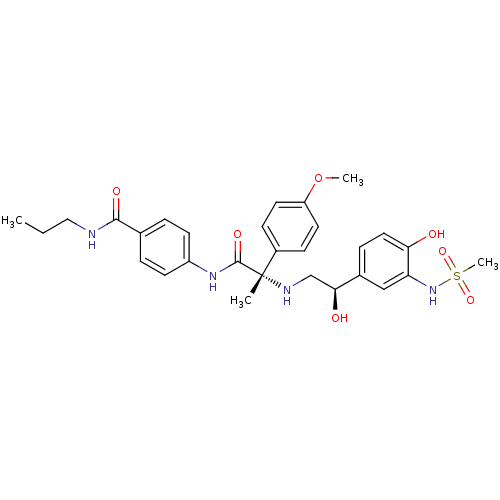 (4-[(S)-2-[2-Hydroxy-2-((R)-4-hydroxy-3-methanesulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human beta-3 adrenergic receptor expressed in CHO cells | Bioorg Med Chem Lett 14: 3525-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.074 BindingDB Entry DOI: 10.7270/Q2MP52Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50106823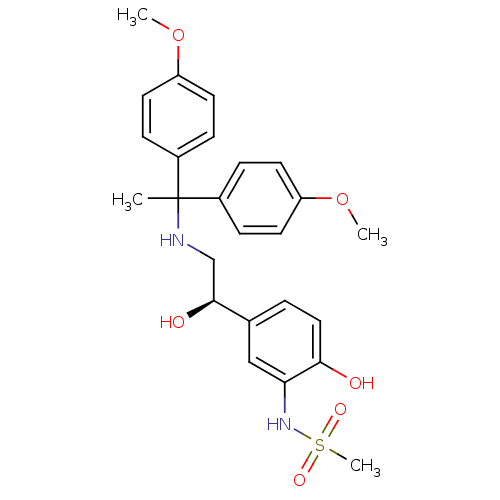 (CHEMBL102222 | N-(5-{(R)-2-[1,1-Bis-(4-methoxy-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against CHO cells transfected with human beta-3 adrenergic receptor in the presence of [125I]iodocyanopindolol | Bioorg Med Chem Lett 11: 3035-9 (2001) BindingDB Entry DOI: 10.7270/Q2MS3T97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 296 total ) | Next | Last >> |