| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetylcholinesterase |
|---|
| Ligand | BDBM50304247 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_605400 (CHEMBL1073394) |
|---|
| IC50 | 47±n/a nM |
|---|
| Citation |  Sheng, R; Lin, X; Zhang, J; Chol, KS; Huang, W; Yang, B; He, Q; Hu, Y Design, synthesis and evaluation of flavonoid derivatives as potent AChE inhibitors. Bioorg Med Chem17:6692-8 (2009) [PubMed] Article Sheng, R; Lin, X; Zhang, J; Chol, KS; Huang, W; Yang, B; He, Q; Hu, Y Design, synthesis and evaluation of flavonoid derivatives as potent AChE inhibitors. Bioorg Med Chem17:6692-8 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetylcholinesterase |
|---|
| Name: | Acetylcholinesterase |
|---|
| Synonyms: | ACES_RAT | Acetylcholinesterase (AChE) | Acetylcholinesterase and butyrylcholinesterase (AChE and BChE) | Acetylcholinesterase precursor | Acetylcholinesterase, AChE | Ache |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 68193.62 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | P37136 |
|---|
| Residue: | 614 |
|---|
| Sequence: | MRPPWYPLHTPSLASPLLFLLLSLLGGGARAEGREDPQLLVRVRGGQLRGIRLKAPGGPV
SAFLGIPFAEPPVGSRRFMPPEPKRPWSGILDATTFQNVCYQYVDTLYPGFEGTEMWNPN
RELSEDCLYLNVWTPYPRPTSPTPVLIWIYGGGFYSGASSLDVYDGRFLAQVEGTVLVSM
NYRVGTFGFLALPGSREAPGNVGLLDQRLALQWVQENIAAFGGDPMSVTLFGESAGAASV
GMHILSLPSRSLFHRAVLQSGTPNGPWATVSAGEARRRATLLARLVGCPPGGAGGNDTEL
ISCLRTRPAQDLVDHEWHVLPQESIFRFSFVPVVDGDFLSDTPDALINTGDFQDLQVLVG
VVKDEGSYFLVYGVPGFSKDNESLISRAQFLAGVRIGVPQASDLAAEAVVLHYTDWLHPE
DPAHLRDAMSAVVGDHNVVCPVAQLAGRLAAQGARVYAYIFEHRASTLTWPLWMGVPHGY
EIEFIFGLPLDPSLNYTVEERIFAQRLMQYWTNFARTGDPNDPRDSKSPRWPPYTTAAQQ
YVSLNLKPLEVRRGLRAQTCAFWNRFLPKLLSATDTLDEAERQWKAEFHRWSSYMVHWKN
QFDHYSKQERCSDL
|
|
|
|---|
| BDBM50304247 |
|---|
| n/a |
|---|
| Name | BDBM50304247 |
|---|
| Synonyms: | (E)-1-(2-Hydroxy-4,5-dimethoxyphenyl)-3-[3-(pyrrolidin-1-ylmethyl)-phenyl]-prop-2-en-1-one | CHEMBL594225 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H23NO4 |
|---|
| Mol. Mass. | 353.4116 |
|---|
| SMILES | COc1cc(O)c(cc1OC)C(=O)\C=C\c1cccc(c1)N1CCCC1 |
|---|
| Structure | 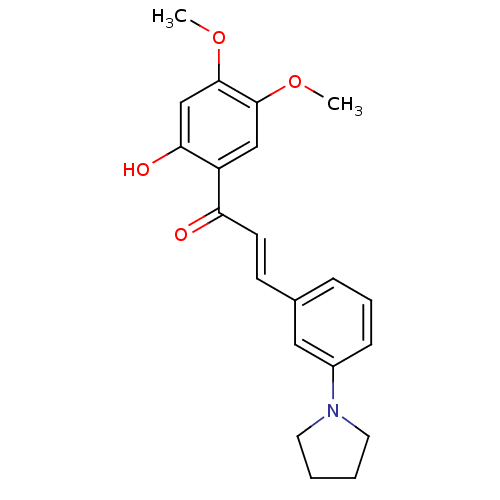
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Sheng, R; Lin, X; Zhang, J; Chol, KS; Huang, W; Yang, B; He, Q; Hu, Y Design, synthesis and evaluation of flavonoid derivatives as potent AChE inhibitors. Bioorg Med Chem17:6692-8 (2009) [PubMed] Article
Sheng, R; Lin, X; Zhang, J; Chol, KS; Huang, W; Yang, B; He, Q; Hu, Y Design, synthesis and evaluation of flavonoid derivatives as potent AChE inhibitors. Bioorg Med Chem17:6692-8 (2009) [PubMed] Article