| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2D6 |
|---|
| Ligand | BDBM50339033 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_729878 (CHEMBL1695232) |
|---|
| IC50 | >25000±n/a nM |
|---|
| Citation |  Xue, CB; Chen, L; Cao, G; Zhang, K; Wang, A; Meloni, D; Glenn, J; Anand, R; Xia, M; Kong, L; Huang, T; Feng, H; Zheng, C; Li, M; Galya, L; Zhou, J; Shin, N; Baribaud, F; Solomon, K; Scherle, P; Zhao, B; Diamond, S; Emm, T; Keller, D; Contel, N; Yeleswaram, S; Vaddi, K; Hollis, G; Newton, R; Friedman, S; Metcalf, B. Discovery of INCB9471, a Potent, Selective, and Orally Bioavailable CCR5 Antagonist with Potent Anti-HIV-1 Activity. ACS Med Chem Lett1:483-487 (2010) [PubMed] Article Xue, CB; Chen, L; Cao, G; Zhang, K; Wang, A; Meloni, D; Glenn, J; Anand, R; Xia, M; Kong, L; Huang, T; Feng, H; Zheng, C; Li, M; Galya, L; Zhou, J; Shin, N; Baribaud, F; Solomon, K; Scherle, P; Zhao, B; Diamond, S; Emm, T; Keller, D; Contel, N; Yeleswaram, S; Vaddi, K; Hollis, G; Newton, R; Friedman, S; Metcalf, B. Discovery of INCB9471, a Potent, Selective, and Orally Bioavailable CCR5 Antagonist with Potent Anti-HIV-1 Activity. ACS Med Chem Lett1:483-487 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2D6 |
|---|
| Name: | Cytochrome P450 2D6 |
|---|
| Synonyms: | CP2D6_HUMAN | CYP2D6 | CYP2DL1 | CYPIID6 | Cytochrome P450 2D6 (CYP2D6) | Debrisoquine 4-hydroxylase | P450-DB1 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 55774.82 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P10635 |
|---|
| Residue: | 497 |
|---|
| Sequence: | MGLEALVPLAVIVAIFLLLVDLMHRRQRWAARYPPGPLPLPGLGNLLHVDFQNTPYCFDQ
LRRRFGDVFSLQLAWTPVVVLNGLAAVREALVTHGEDTADRPPVPITQILGFGPRSQGVF
LARYGPAWREQRRFSVSTLRNLGLGKKSLEQWVTEEAACLCAAFANHSGRPFRPNGLLDK
AVSNVIASLTCGRRFEYDDPRFLRLLDLAQEGLKEESGFLREVLNAVPVLLHIPALAGKV
LRFQKAFLTQLDELLTEHRMTWDPAQPPRDLTEAFLAEMEKAKGNPESSFNDENLRIVVA
DLFSAGMVTTSTTLAWGLLLMILHPDVQRRVQQEIDDVIGQVRRPEMGDQAHMPYTTAVI
HEVQRFGDIVPLGVTHMTSRDIEVQGFRIPKGTTLITNLSSVLKDEAVWEKPFRFHPEHF
LDAQGHFVKPEAFLPFSAGRRACLGEPLARMELFLFFTSLLQHFSFSVPTGQPRPSHHGV
FAFLVSPSPYELCAVPR
|
|
|
|---|
| BDBM50339033 |
|---|
| n/a |
|---|
| Name | BDBM50339033 |
|---|
| Synonyms: | 5-[(4-(3S)-4-[(1R,2R)-2-ethoxy-5-(trifluoromethyl)-2,3-dihydro-1H-inden-1-yl]-3-methylpiperazin-1-yl-4-methylpiperidin-1-yl)carbonyl]-4,6-dimethylpyrimidine | CHEMBL1688243 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C30H40F3N5O2 |
|---|
| Mol. Mass. | 559.6661 |
|---|
| SMILES | CCO[C@@H]1Cc2cc(ccc2[C@H]1N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C)C(F)(F)F |r| |
|---|
| Structure | 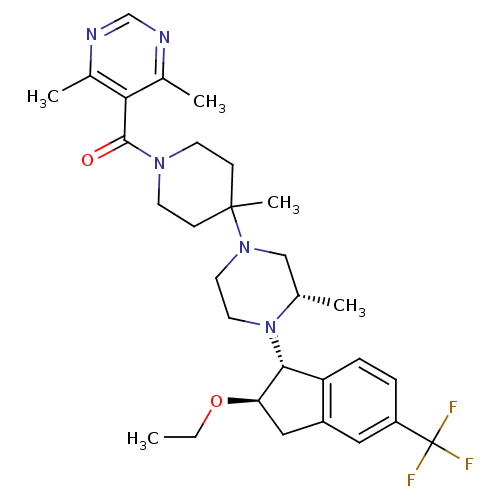
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Xue, CB; Chen, L; Cao, G; Zhang, K; Wang, A; Meloni, D; Glenn, J; Anand, R; Xia, M; Kong, L; Huang, T; Feng, H; Zheng, C; Li, M; Galya, L; Zhou, J; Shin, N; Baribaud, F; Solomon, K; Scherle, P; Zhao, B; Diamond, S; Emm, T; Keller, D; Contel, N; Yeleswaram, S; Vaddi, K; Hollis, G; Newton, R; Friedman, S; Metcalf, B. Discovery of INCB9471, a Potent, Selective, and Orally Bioavailable CCR5 Antagonist with Potent Anti-HIV-1 Activity. ACS Med Chem Lett1:483-487 (2010) [PubMed] Article
Xue, CB; Chen, L; Cao, G; Zhang, K; Wang, A; Meloni, D; Glenn, J; Anand, R; Xia, M; Kong, L; Huang, T; Feng, H; Zheng, C; Li, M; Galya, L; Zhou, J; Shin, N; Baribaud, F; Solomon, K; Scherle, P; Zhao, B; Diamond, S; Emm, T; Keller, D; Contel, N; Yeleswaram, S; Vaddi, K; Hollis, G; Newton, R; Friedman, S; Metcalf, B. Discovery of INCB9471, a Potent, Selective, and Orally Bioavailable CCR5 Antagonist with Potent Anti-HIV-1 Activity. ACS Med Chem Lett1:483-487 (2010) [PubMed] Article