

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM13465 ((2S)-N-[(1S)-1-carbamoyl-2-{4-[(5S)-1,1,3-trioxo-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition constant against protein-tyrosine phosphatase 1B by PNPP enzyme assay | J Med Chem 48: 6544-8 (2005) Article DOI: 10.1021/jm0504555 BindingDB Entry DOI: 10.7270/Q2805252 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM26810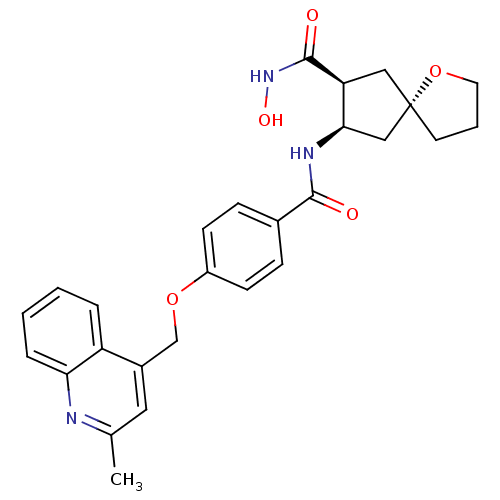 ((5S,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.25E+3 | -33.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM26809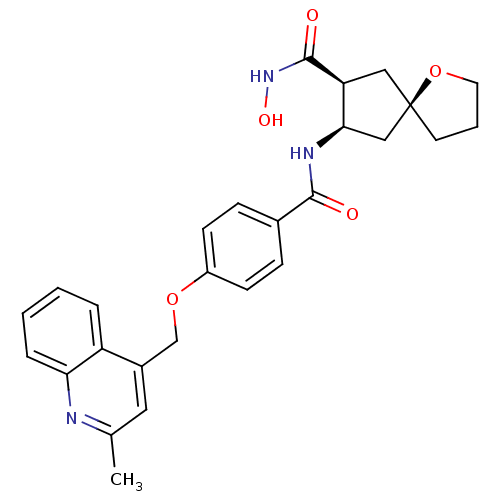 ((5R,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.36E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM26809 ((5R,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.45E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM26810 ((5S,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.92E+3 | -32.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM26808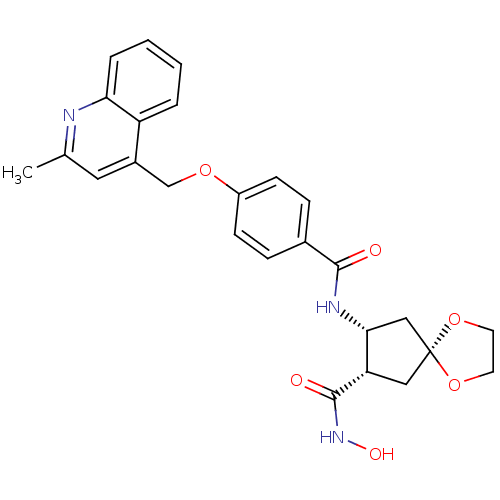 ((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.13E+3 | >-32.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM26808 ((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | -31.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM26809 ((5R,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >4.95E+3 | >-30.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM26808 ((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >4.95E+3 | >-30.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM26810 ((5S,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >4.95E+3 | >-30.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Sus scrofa (pig)) | BDBM26808 ((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Sus scrofa (pig)) | BDBM26809 ((5R,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Sus scrofa (pig)) | BDBM26810 ((5S,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50363942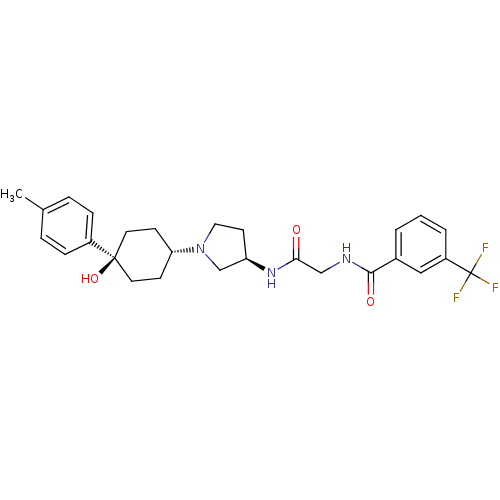 (CHEMBL1951766) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis after 30 mins | ACS Med Chem Lett 2: 450-454 (2011) Article DOI: 10.1021/ml200030q BindingDB Entry DOI: 10.7270/Q2WD4116 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50363942 (CHEMBL1951766) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from CCR2 in human PBMC after 30 mins by gamma counter | ACS Med Chem Lett 2: 450-454 (2011) Article DOI: 10.1021/ml200030q BindingDB Entry DOI: 10.7270/Q2WD4116 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50339029 (5-({4-[(3S)-4-(5-Bromo-2,3-dihydro-1H-inden-1-yl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in IL-10 stimulated human PBMC cells assessed as MIP-1beta induced chemotaxis | ACS Med Chem Lett 1: 483-487 (2010) Article DOI: 10.1021/ml1001536 BindingDB Entry DOI: 10.7270/Q2SQ90PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50339030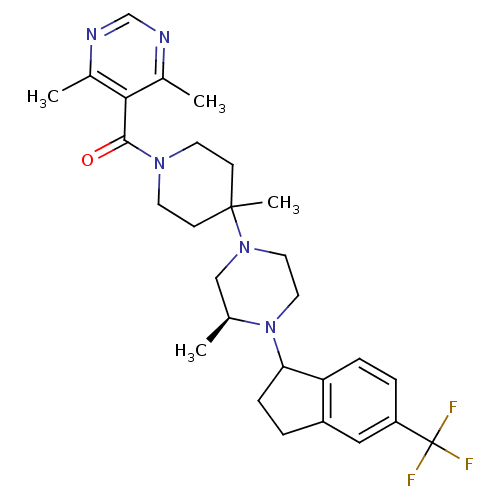 (4,6-Dimethyl-5-[(4-methyl-4-{(3S)-3-methyl-4-[5-(t...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in IL-10 stimulated human PBMC cells assessed as MIP-1beta induced chemotaxis | ACS Med Chem Lett 1: 483-487 (2010) Article DOI: 10.1021/ml1001536 BindingDB Entry DOI: 10.7270/Q2SQ90PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145685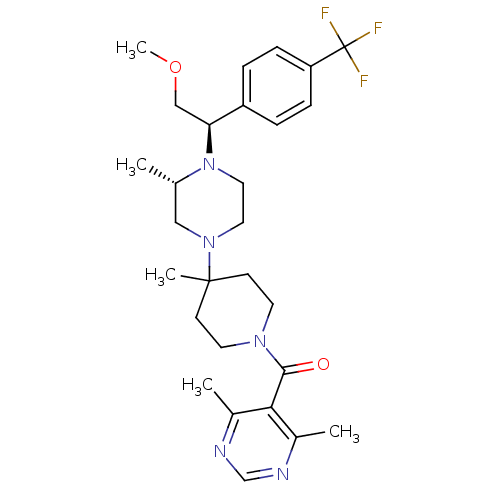 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(R)-2-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in IL-10 stimulated human PBMC cells assessed as MIP-1beta induced chemotaxis | ACS Med Chem Lett 1: 483-487 (2010) Article DOI: 10.1021/ml1001536 BindingDB Entry DOI: 10.7270/Q2SQ90PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50339033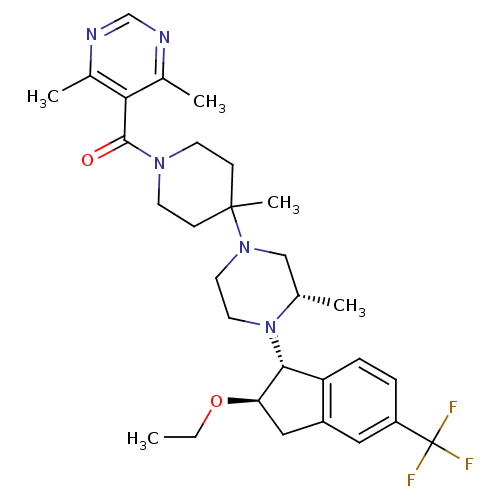 (5-[(4-(3S)-4-[(1R,2R)-2-ethoxy-5-(trifluoromethyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 assessed as inhibition of receptor internalization | ACS Med Chem Lett 1: 483-487 (2010) Article DOI: 10.1021/ml1001536 BindingDB Entry DOI: 10.7270/Q2SQ90PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50337608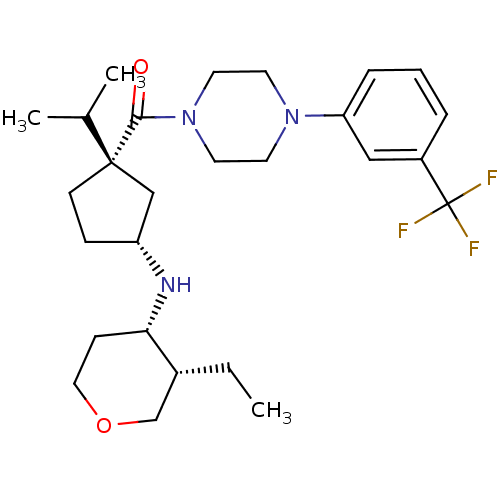 (CHEMBL1683063 | cis-((1S,3R)-3-(3-ethyl-tetrahydro...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis after 30 mins | Bioorg Med Chem Lett 21: 1442-6 (2011) Article DOI: 10.1016/j.bmcl.2011.01.015 BindingDB Entry DOI: 10.7270/Q22J6C4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50363952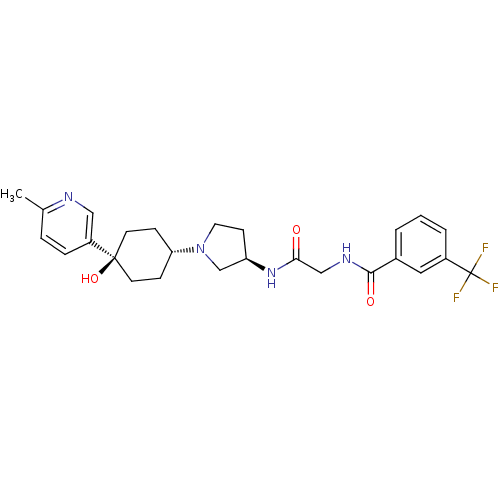 (CHEMBL1951777) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from CCR2 in human PBMC after 30 mins by gamma counter | ACS Med Chem Lett 2: 450-454 (2011) Article DOI: 10.1021/ml200030q BindingDB Entry DOI: 10.7270/Q2WD4116 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50337604 (CHEMBL1683059 | Cis-((1S,3R)-1-isopropyl-3-(3-meth...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis after 30 mins | Bioorg Med Chem Lett 21: 1442-6 (2011) Article DOI: 10.1016/j.bmcl.2011.01.015 BindingDB Entry DOI: 10.7270/Q22J6C4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50363953 (CHEMBL1951778 | CHEMBL1963131) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CCR2-mediated Erk phosphorylation | ACS Med Chem Lett 2: 450-454 (2011) Article DOI: 10.1021/ml200030q BindingDB Entry DOI: 10.7270/Q2WD4116 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50363944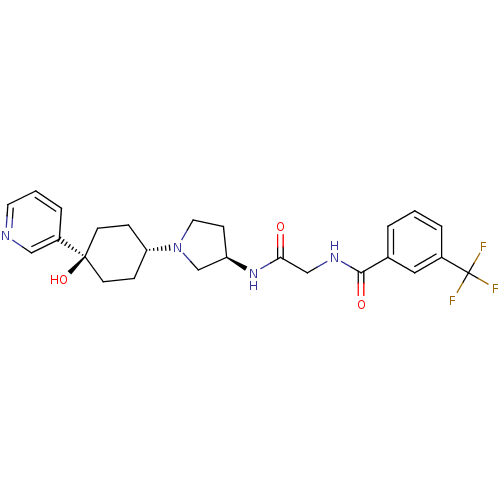 (CHEMBL1951769) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from CCR2 in human PBMC after 30 mins by gamma counter | ACS Med Chem Lett 2: 450-454 (2011) Article DOI: 10.1021/ml200030q BindingDB Entry DOI: 10.7270/Q2WD4116 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50363954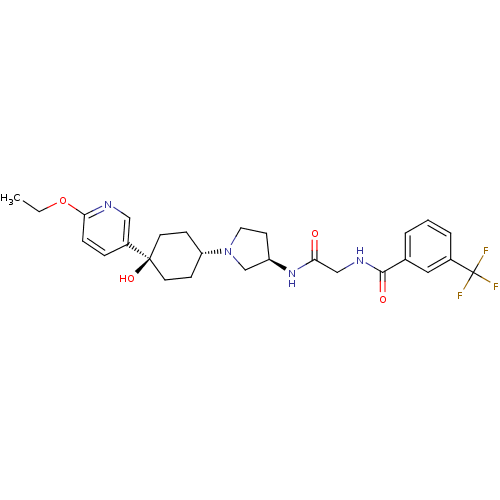 (CHEMBL1951779) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis after 30 mins | ACS Med Chem Lett 2: 450-454 (2011) Article DOI: 10.1021/ml200030q BindingDB Entry DOI: 10.7270/Q2WD4116 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Mus musculus) | BDBM50337619 (((1S,3R)-1-isopropyl-3-((3S,4S)-3-methoxytetrahydr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Binding affinity to mouse CCR2 | Bioorg Med Chem Lett 21: 1442-6 (2011) Article DOI: 10.1016/j.bmcl.2011.01.015 BindingDB Entry DOI: 10.7270/Q22J6C4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Rattus norvegicus) | BDBM50337619 (((1S,3R)-1-isopropyl-3-((3S,4S)-3-methoxytetrahydr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Binding affinity to rat CCR2 | Bioorg Med Chem Lett 21: 1442-6 (2011) Article DOI: 10.1016/j.bmcl.2011.01.015 BindingDB Entry DOI: 10.7270/Q22J6C4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50363945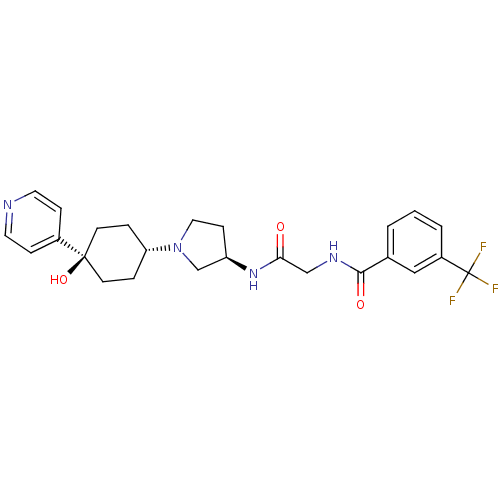 (CHEMBL1951770) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis after 30 mins | ACS Med Chem Lett 2: 450-454 (2011) Article DOI: 10.1021/ml200030q BindingDB Entry DOI: 10.7270/Q2WD4116 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50337619 (((1S,3R)-1-isopropyl-3-((3S,4S)-3-methoxytetrahydr...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from human CCR2 after 30 mins by gamma counting | Bioorg Med Chem Lett 21: 1442-6 (2011) Article DOI: 10.1016/j.bmcl.2011.01.015 BindingDB Entry DOI: 10.7270/Q22J6C4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50339033 (5-[(4-(3S)-4-[(1R,2R)-2-ethoxy-5-(trifluoromethyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 assessed as inhibition of ERK phosphorylation | ACS Med Chem Lett 1: 483-487 (2010) Article DOI: 10.1021/ml1001536 BindingDB Entry DOI: 10.7270/Q2SQ90PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50339033 (5-[(4-(3S)-4-[(1R,2R)-2-ethoxy-5-(trifluoromethyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in IL-10 stimulated human PBMC cells assessed as MIP-1beta induced chemotaxis | ACS Med Chem Lett 1: 483-487 (2010) Article DOI: 10.1021/ml1001536 BindingDB Entry DOI: 10.7270/Q2SQ90PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145685 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(R)-2-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]MIP-1beta from CCR5 in IL-10-stimulated human monocytes | ACS Med Chem Lett 1: 483-487 (2010) Article DOI: 10.1021/ml1001536 BindingDB Entry DOI: 10.7270/Q2SQ90PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50337604 (CHEMBL1683059 | Cis-((1S,3R)-1-isopropyl-3-(3-meth...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from human CCR2 after 30 mins by gamma counting | Bioorg Med Chem Lett 21: 1442-6 (2011) Article DOI: 10.1016/j.bmcl.2011.01.015 BindingDB Entry DOI: 10.7270/Q22J6C4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50363943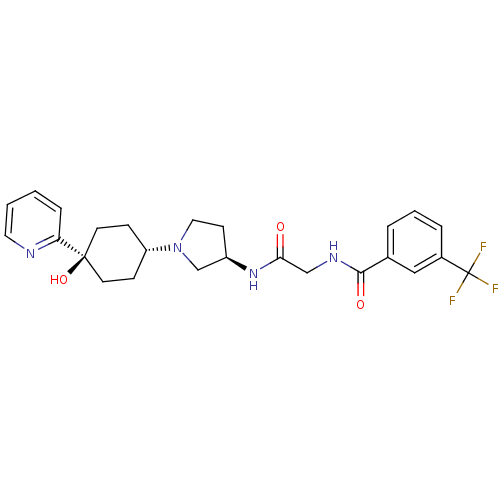 (CHEMBL1951768) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis after 30 mins | ACS Med Chem Lett 2: 450-454 (2011) Article DOI: 10.1021/ml200030q BindingDB Entry DOI: 10.7270/Q2WD4116 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50337634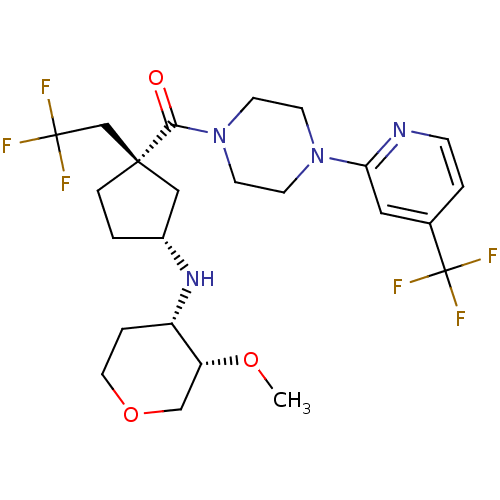 (((1S,3R)-3-((3S,4S)-3-methoxy-tetrahydro-2H-pyran-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Displacement of MCP-Alexa 488 from CCR2 in human whole blood after 5 mins by flow cytometry | Bioorg Med Chem Lett 21: 1442-6 (2011) Article DOI: 10.1016/j.bmcl.2011.01.015 BindingDB Entry DOI: 10.7270/Q22J6C4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50337619 (((1S,3R)-1-isopropyl-3-((3S,4S)-3-methoxytetrahydr...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis after 30 mins | Bioorg Med Chem Lett 21: 1442-6 (2011) Article DOI: 10.1016/j.bmcl.2011.01.015 BindingDB Entry DOI: 10.7270/Q22J6C4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50363954 (CHEMBL1951779) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from CCR2 in human PBMC after 30 mins by gamma counter | ACS Med Chem Lett 2: 450-454 (2011) Article DOI: 10.1021/ml200030q BindingDB Entry DOI: 10.7270/Q2WD4116 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50363943 (CHEMBL1951768) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from CCR2 in human PBMC after 30 mins by gamma counter | ACS Med Chem Lett 2: 450-454 (2011) Article DOI: 10.1021/ml200030q BindingDB Entry DOI: 10.7270/Q2WD4116 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50337634 (((1S,3R)-3-((3S,4S)-3-methoxy-tetrahydro-2H-pyran-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from human CCR2 after 30 mins by gamma counting | Bioorg Med Chem Lett 21: 1442-6 (2011) Article DOI: 10.1016/j.bmcl.2011.01.015 BindingDB Entry DOI: 10.7270/Q22J6C4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50339032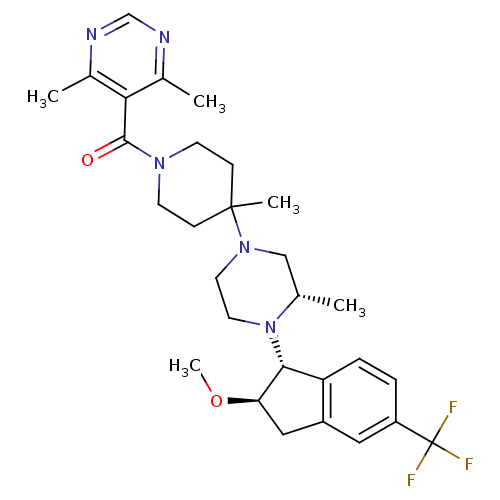 (5-[(4-(3S)-4-[(1R,2R)-2-methoxy-5-(trifluoromethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in IL-10 stimulated human PBMC cells assessed as MIP-1beta induced chemotaxis | ACS Med Chem Lett 1: 483-487 (2010) Article DOI: 10.1021/ml1001536 BindingDB Entry DOI: 10.7270/Q2SQ90PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50363953 (CHEMBL1951778 | CHEMBL1963131) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from CCR2 in human PBMC after 30 mins by gamma counter | ACS Med Chem Lett 2: 450-454 (2011) Article DOI: 10.1021/ml200030q BindingDB Entry DOI: 10.7270/Q2WD4116 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50363944 (CHEMBL1951769) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis after 30 mins | ACS Med Chem Lett 2: 450-454 (2011) Article DOI: 10.1021/ml200030q BindingDB Entry DOI: 10.7270/Q2WD4116 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50339029 (5-({4-[(3S)-4-(5-Bromo-2,3-dihydro-1H-inden-1-yl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]MIP-1beta from CCR5 in IL-10-stimulated human monocytes | ACS Med Chem Lett 1: 483-487 (2010) Article DOI: 10.1021/ml1001536 BindingDB Entry DOI: 10.7270/Q2SQ90PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50337619 (((1S,3R)-1-isopropyl-3-((3S,4S)-3-methoxytetrahydr...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Displacement of MCP-Alexa 488 from CCR2 in human whole blood after 5 mins by flow cytometry | Bioorg Med Chem Lett 21: 1442-6 (2011) Article DOI: 10.1016/j.bmcl.2011.01.015 BindingDB Entry DOI: 10.7270/Q22J6C4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50339029 (5-({4-[(3S)-4-(5-Bromo-2,3-dihydro-1H-inden-1-yl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]MIP-1beta from CCR5 in IL-10-stimulated human monocytes | ACS Med Chem Lett 1: 483-487 (2010) Article DOI: 10.1021/ml1001536 BindingDB Entry DOI: 10.7270/Q2SQ90PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50337619 (((1S,3R)-1-isopropyl-3-((3S,4S)-3-methoxytetrahydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Displacement of labeled MIP-1beta from human CCR5 receptor | Bioorg Med Chem Lett 21: 1442-6 (2011) Article DOI: 10.1016/j.bmcl.2011.01.015 BindingDB Entry DOI: 10.7270/Q22J6C4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50363945 (CHEMBL1951770) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from CCR2 in human PBMC after 30 mins by gamma counter | ACS Med Chem Lett 2: 450-454 (2011) Article DOI: 10.1021/ml200030q BindingDB Entry DOI: 10.7270/Q2WD4116 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50337608 (CHEMBL1683063 | cis-((1S,3R)-3-(3-ethyl-tetrahydro...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from human CCR2 after 30 mins by gamma counting | Bioorg Med Chem Lett 21: 1442-6 (2011) Article DOI: 10.1016/j.bmcl.2011.01.015 BindingDB Entry DOI: 10.7270/Q22J6C4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50339032 (5-[(4-(3S)-4-[(1R,2R)-2-methoxy-5-(trifluoromethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]MIP-1beta from CCR5 in IL-10-stimulated human monocytes | ACS Med Chem Lett 1: 483-487 (2010) Article DOI: 10.1021/ml1001536 BindingDB Entry DOI: 10.7270/Q2SQ90PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50363953 (CHEMBL1951778 | CHEMBL1963131) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis after 30 mins | ACS Med Chem Lett 2: 450-454 (2011) Article DOI: 10.1021/ml200030q BindingDB Entry DOI: 10.7270/Q2WD4116 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 174 total ) | Next | Last >> |