| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetylcholinesterase |
|---|
| Ligand | BDBM50346444 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_751131 (CHEMBL1787068) |
|---|
| IC50 | 9±n/a nM |
|---|
| Citation |  Tasso, B; Catto, M; Nicolotti, O; Novelli, F; Tonelli, M; Giangreco, I; Pisani, L; Sparatore, A; Boido, V; Carotti, A; Sparatore, F Quinolizidinyl derivatives of bi- and tricyclic systems as potent inhibitors of acetyl- and butyrylcholinesterase with potential in Alzheimer's disease. Eur J Med Chem46:2170-84 (2011) [PubMed] Article Tasso, B; Catto, M; Nicolotti, O; Novelli, F; Tonelli, M; Giangreco, I; Pisani, L; Sparatore, A; Boido, V; Carotti, A; Sparatore, F Quinolizidinyl derivatives of bi- and tricyclic systems as potent inhibitors of acetyl- and butyrylcholinesterase with potential in Alzheimer's disease. Eur J Med Chem46:2170-84 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetylcholinesterase |
|---|
| Name: | Acetylcholinesterase |
|---|
| Synonyms: | ACES_HUMAN | ACHE | Acetylcholinesterase (AChE) | Acetylcholinesterase (human AChE) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 67792.70 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P22303 |
|---|
| Residue: | 614 |
|---|
| Sequence: | MRPPQCLLHTPSLASPLLLLLLWLLGGGVGAEGREDAELLVTVRGGRLRGIRLKTPGGPV
SAFLGIPFAEPPMGPRRFLPPEPKQPWSGVVDATTFQSVCYQYVDTLYPGFEGTEMWNPN
RELSEDCLYLNVWTPYPRPTSPTPVLVWIYGGGFYSGASSLDVYDGRFLVQAERTVLVSM
NYRVGAFGFLALPGSREAPGNVGLLDQRLALQWVQENVAAFGGDPTSVTLFGESAGAASV
GMHLLSPPSRGLFHRAVLQSGAPNGPWATVGMGEARRRATQLAHLVGCPPGGTGGNDTEL
VACLRTRPAQVLVNHEWHVLPQESVFRFSFVPVVDGDFLSDTPEALINAGDFHGLQVLVG
VVKDEGSYFLVYGAPGFSKDNESLISRAEFLAGVRVGVPQVSDLAAEAVVLHYTDWLHPE
DPARLREALSDVVGDHNVVCPVAQLAGRLAAQGARVYAYVFEHRASTLSWPLWMGVPHGY
EIEFIFGIPLDPSRNYTAEEKIFAQRLMRYWANFARTGDPNEPRDPKAPQWPPYTAGAQQ
YVSLDLRPLEVRRGLRAQACAFWNRFLPKLLSATDTLDEAERQWKAEFHRWSSYMVHWKN
QFDHYSKQDRCSDL
|
|
|
|---|
| BDBM50346444 |
|---|
| n/a |
|---|
| Name | BDBM50346444 |
|---|
| Synonyms: | CHEMBL1782707 | [4-(4-morpholinyl)butyl]carbamic acid-3-(1,1-dimethyl-2-dimethylaminoethyl)phenylester |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H35N3O3 |
|---|
| Mol. Mass. | 377.5209 |
|---|
| SMILES | CN(C)CC(C)(C)c1cccc(OC(=O)NCCCCN2CCOCC2)c1 |
|---|
| Structure | 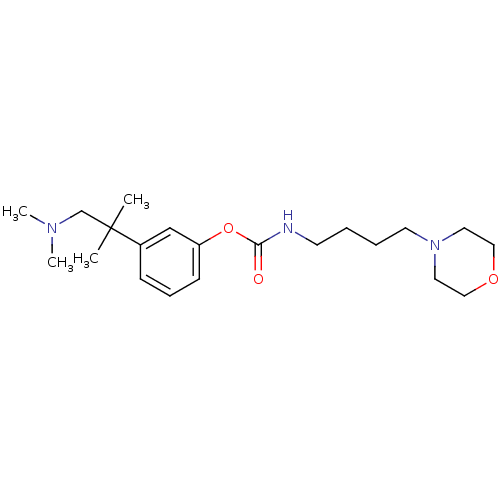
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tasso, B; Catto, M; Nicolotti, O; Novelli, F; Tonelli, M; Giangreco, I; Pisani, L; Sparatore, A; Boido, V; Carotti, A; Sparatore, F Quinolizidinyl derivatives of bi- and tricyclic systems as potent inhibitors of acetyl- and butyrylcholinesterase with potential in Alzheimer's disease. Eur J Med Chem46:2170-84 (2011) [PubMed] Article
Tasso, B; Catto, M; Nicolotti, O; Novelli, F; Tonelli, M; Giangreco, I; Pisani, L; Sparatore, A; Boido, V; Carotti, A; Sparatore, F Quinolizidinyl derivatives of bi- and tricyclic systems as potent inhibitors of acetyl- and butyrylcholinesterase with potential in Alzheimer's disease. Eur J Med Chem46:2170-84 (2011) [PubMed] Article