| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tyrosine-protein phosphatase non-receptor type 1 |
|---|
| Ligand | BDBM50362198 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_798051 (CHEMBL1943106) |
|---|
| IC50 | 38±n/a nM |
|---|
| Citation |  Patel, D; Jain, M; Shah, SR; Bahekar, R; Jadav, P; Joharapurkar, A; Dhanesha, N; Shaikh, M; Sairam, KV; Kapadnis, P Discovery of potent, selective and orally bioavailable triaryl-sulfonamide based PTP1B inhibitors. Bioorg Med Chem Lett22:1111-7 (2012) [PubMed] Article Patel, D; Jain, M; Shah, SR; Bahekar, R; Jadav, P; Joharapurkar, A; Dhanesha, N; Shaikh, M; Sairam, KV; Kapadnis, P Discovery of potent, selective and orally bioavailable triaryl-sulfonamide based PTP1B inhibitors. Bioorg Med Chem Lett22:1111-7 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Tyrosine-protein phosphatase non-receptor type 1 |
|---|
| Name: | Tyrosine-protein phosphatase non-receptor type 1 |
|---|
| Synonyms: | PTN1_HUMAN | PTP1B | PTPN1 | Protein tyrosine phosphatase 1B (PTP1B) | Protein tyrosine phosphatase-1B (PTP1B) | Protein-tyrosine phosphatase 1B | Protein-tyrosine phosphatase 1B (PTP1B) | Tyrosine-protein phosphatase non-receptor type 1 | Tyrosine-protein phosphatase non-receptor type 1 (PTP1B) |
|---|
| Type: | Protein phosphatase |
|---|
| Mol. Mass.: | 49963.76 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Human recombinant GST-fusion PTP1B (1-435). |
|---|
| Residue: | 435 |
|---|
| Sequence: | MEMEKEFEQIDKSGSWAAIYQDIRHEASDFPCRVAKLPKNKNRNRYRDVSPFDHSRIKLH
QEDNDYINASLIKMEEAQRSYILTQGPLPNTCGHFWEMVWEQKSRGVVMLNRVMEKGSLK
CAQYWPQKEEKEMIFEDTNLKLTLISEDIKSYYTVRQLELENLTTQETREILHFHYTTWP
DFGVPESPASFLNFLFKVRESGSLSPEHGPVVVHCSAGIGRSGTFCLADTCLLLMDKRKD
PSSVDIKKVLLEMRKFRMGLIQTADQLRFSYLAVIEGAKFIMGDSSVQDQWKELSHEDLE
PPPEHIPPPPRPPKRILEPHNGKCREFFPNHQWVKEETQEDKDCPIKEEKGSPLNAAPYG
IESMSQDTEVRSRVVGGSLRGAQAASPAKGEPSLPEKDEDHALSYWKPFLVNMCVATVLT
AGAYLCYRFLFNSNT
|
|
|
|---|
| BDBM50362198 |
|---|
| n/a |
|---|
| Name | BDBM50362198 |
|---|
| Synonyms: | CHEMBL1938831 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H22F3N5O7S3 |
|---|
| Mol. Mass. | 693.694 |
|---|
| SMILES | NS(=O)(=O)C(F)(F)c1cc2nc(CN(Cc3ccc(cc3)-c3csnn3)S(=O)(=O)c3ccc(OCC(O)=O)cc3)ccc2cc1F |
|---|
| Structure | 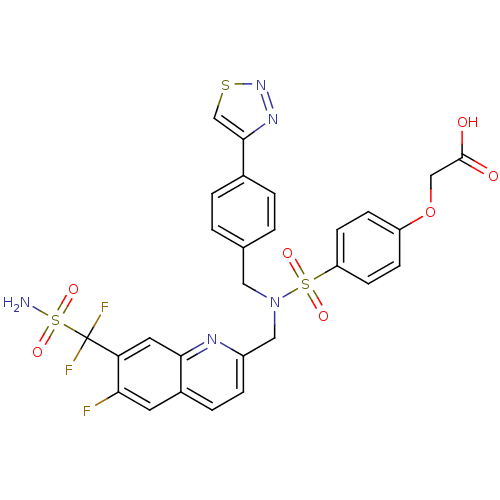
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Patel, D; Jain, M; Shah, SR; Bahekar, R; Jadav, P; Joharapurkar, A; Dhanesha, N; Shaikh, M; Sairam, KV; Kapadnis, P Discovery of potent, selective and orally bioavailable triaryl-sulfonamide based PTP1B inhibitors. Bioorg Med Chem Lett22:1111-7 (2012) [PubMed] Article
Patel, D; Jain, M; Shah, SR; Bahekar, R; Jadav, P; Joharapurkar, A; Dhanesha, N; Shaikh, M; Sairam, KV; Kapadnis, P Discovery of potent, selective and orally bioavailable triaryl-sulfonamide based PTP1B inhibitors. Bioorg Med Chem Lett22:1111-7 (2012) [PubMed] Article